Abstract
Cancer-associated fibroblasts (CAFs) are one of the most prominent and active components in the pancreatic tumor microenvironment. Our data show that CAFs are critical for survival from pancreatic ductal adenocarcinoma (PDAC) on glutamine deprivation. Specifically, we uncovered a role for nucleosides, which are secreted by CAFs through autophagy in a nuclear fragile X mental retardation-interacting protein 1 (NUFIP1)-dependent manner, increased glucose utilization and promoted growth of PDAC. Moreover, we demonstrate that CAF-derived nucleosides induced glucose consumption under glutamine-deprived conditions and displayed a dependence on MYC. Using an orthotopic mouse model of PDAC, we found that inhibiting nucleoside secretion by targeting NUFIP1 in the stroma reduced tumor weight. This finding highlights a previously unappreciated metabolic network within pancreatic tumors in which diverse nutrients are used to promote growth in an austere tumor microenvironment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The detailed results from the RNA-sequencing experiments are deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (accession no. GSE185750). Metabolomics data have been deposited into MassIVE under accession no. MSV000089717. Source data are provided with this paper. All other data supporting the findings of the present study are available from the corresponding author on reasonable request.
References
Pereira, B. A. et al. CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. Trends Cancer 5, 724–741 (2019).
Hosein, A. N., Brekken, R. A. & Maitra, A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastro. Hepatol. 17, 487–505 (2020).
Neuzillet, C. et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J. Pathol. 248, 51–65 (2019).
Klionsky, D. J. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8, 931–937 (2007).
Mizushima, N. Autophagy in protein and organelle turnover. Cold Spring Harb. Symp. Quant. Biol. 76, 397–40 (2011).
Endo, S. et al. Autophagy Is required for activation of pancreatic stellate cells, associated with pancreatic cancer progression and promotes growth of pancreatic tumors in mice. Gastroenterology 152, 1492–1506.e1424 (2017).
Sousa, C. M. et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536, 479–483 (2016).
Zhang, X. B. et al. Blocking autophagy in cancer-associated fibroblasts supports chemotherapy of pancreatic cancer cells. Front. Oncol. 8, 590 (2018).
Goruppi, S. et al. Autophagy controls CSL/RBPJ kappa stability through a p62/SQSTM1-dependent mechanism. Cell Rep, 24, 3108–310 (2018).
Zhao, X. L. et al. High-mobility group box 1 released by autophagic cancer-associated fibroblasts maintains the stemness of luminal breast cancer cells. J. Pathol. 243, 376–389 (2017).
Gatica, D., Lahiri, V. & Klionsky, D. J. Cargo recognition and degradation by selective autophagy. Nat. Cell Biol. 20, 233–242 (2018).
Beese, C.J., Brynjolfsdottir, S.H. & Frankel, L.B. Selective autophagy of the protein homeostasis machinery: ribophagy, proteaphagy and ER-phagy. Front. Cell Dev. Biol. 7, 373 (2020).
Wyant, G. A. et al. NUFIP1 is a ribosome receptor for starvation-induced ribophagy. Science 360, 751–758 (2018).
Biancur, D. E. et al. Compensatory metabolic networks in pancreatic cancers upon perturbation of glutamine metabolism. Nat. Commun. 8, 15965 (2017).
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013).
Lee, S. W. et al. EGFR-Pak signaling selectively regulates glutamine deprivation-induced macropinocytosis. Dev. Cell 50, 381–392.e385 (2019).
An, H., Ordureau, A., Korner, M., Paulo, J. A. & Harper, J. W. Systematic quantitative analysis of ribosome inventory during nutrient stress. Nature 583, 303–309 (2020).
Bardoni, B., Schenck, A. & Mandel, J. L. A novel RNA-binding nuclear protein that interacts with the fragile X mental retardation (FMR1) protein. Hum Mol Genet 8, 2557–2566 (1999).
Itakura, E., Kishi-Itakura, C. & Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151, 1256–1269 (2012).
Byun, J. K. et al. Oncogenic KRAS signaling activates mTORC1 through COUP-TFII-mediated lactate production. EMBO Rep 20, e47451 (2019).
Kaadige, M. R., Looper, R. E., Kamalanaadhan, S. & Ayer, D. E. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc. Natl Acad. Sci. USA 106, 14878–14883 (2009).
Hsieh, A. L., Walton, Z. E., Altman, B. J., Stine, Z. E. & Dang, C. V. MYC and metabolism on the path to cancer. Semin. Cell Dev. Biol. 43, 11–21 (2015).
Dejure, F. R. et al. The MYC mRNA 3′-UTR couples RNA polymerase II function to glutamine and ribonucleotide levels. EMBO J. 36, 1854–1868 (2017).
Rudnick, J. A. et al. Autophagy in stromal fibroblasts promotes tumor desmoplasia and mammary tumorigenesis. Genes Dev. 35, 963–975 (2021).
Lyssiotis, C. A. & Kimmelman, A. C. Metabolic interactions in the tumor mcroenvironment. Trends Cell Biol. 27, 873–885 (2017).
Yang, L. F. et al. Targeting stromal glutamine synthetase in tumors disrupts tumor microenvironment-regulated cancer cell growth. Cell Metab. 24, 685–700 (2016).
Mishra, R. et al. Stromal epigenetic alterations drive metabolic and neuroendocrine prostate cancer reprogramming. J. Clin. Invest. 128, 4472–4484 (2018).
Zhu, Z. W. et al. Tumour-reprogrammed stromal BCAT1 fuels branched-chain ketoacid dependency in stromal-rich PDAC tumours. Nat. Metab. 2, 775–77 (2020).
Blobel, G. & Potter, V. R. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J. Mol. Biol. 26, 279–27 (1967).
Tajan, M. et al. A role for p53 in the adaptation to glutamine starvation through the expression of SLC1A3. Cell Metab. 28, 721–72 (2018).
Tardito, S. et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat. Cell Biol. 17, 1556–1568 (2015).
Linker, W., Loffler, M. & Schneider, F. Uridine, but not cytidine can sustain growth of Ehrlich ascites tumor cells in glucose-deprived medium with altered proliferation kinetics. Eur. J. Cell Biol. 36, 176–181 (1985).
Hessmann, E., Schneider, G., Ellenrieder, V. & Siveke, J. T. MYC in pancreatic cancer: novel mechanistic insights and their translation into therapeutic strategies. Oncogene 35, 1609–1618 (2016).
Goetzman, E. S. & Prochownik, E. V. The role for Myc in coordinating glycolysis, oxidative phosphorylation, glutaminolysis, and fatty acid metabolism in normal and neoplastic tissues. Front. Endocrinol. 9, 129 (2018).
Wise, D. R. et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl Acad. Sci. USA 105, 18782–18787 (2008).
Lao-On, U. et al. c-Myc directly targets an over-expression of pyruvate carboxylase in highly invasive breast cancer. Biochem. Biophys. Acta Mol. Basis Dis. 1866, 165656 (2020).
Yuneva, M., Zamboni, N., Oefner, P., Sachidanandam, R. & Lazebnik, Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell Biol. 178, 93–105 (2007).
Aguirre, A. J. et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 17, 3112–3126 (2003).
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012).
Hynes, J. et al. Investigation of drug-induced mitochondrial toxicity using fluorescence-based oxygen-sensitive probes. Toxicol. Sci. 92, 186–200 (2006).
Uematsu, M., Nishimura, T., Sakamaki, Y., Yamamoto, H. & Mizushima, N. Accumulation of undegraded autophagosomes by expression of dominant-negative STX17 (syntaxin 17) mutants. Autophagy 13, 1452–1464 (2017).
Sullivan, M. R., Lewis, C. A. & Muir, A. Isolation and quantification of metabolite levels in murine tumor interstitial fluid by LC/MS. Bio. Protoc. 9, e3427 (2019).
Acknowledgements
The present study was supported by the National Key R&D Program of China (nos. 2017YFA0503900 and 2019YFC1005200 to Y.Z.), National Natural Science Foundation of China (nos. 82173020, 81874145 and 81672712 to Y.Z.) and Peking University (nos. BMU2022XKQ004 and PKU2020LCXQ024 to Y.Z.).
Author information
Authors and Affiliations
Contributions
M.Y. carried out in vitro cell experiments, in vivo experiments, manuscript preparation, IHC analysis and statistical analysis. T.B., N.Z., M.F., J.B., W.W., Z.S. S.H., J.Z. and L.W. carried out in vitro cell experiments and in vivo experiments. H.P., K.Y. and Z.H. carried out metabolism experiments and analyzed the data. H.L. and D.F. provided clinical specimens. C.D. contributed to manuscript preparation. W.G.Z supervised the study. Y.Z. conceived, designed and supervised the study and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 PDAC cells were sensitive to glutamine starvation.
(a) Cell growth rate was detected by MTT assay. 14837 T, 14838 T, 8988 T, 8988 S, MIA Paca-2, PL45, HPAC, ASPC, PANC 03.27 and PANC-1 cells were incubated in complete media (25 mM glucose, 4 mM glutamine), no glucose media or no glutamine media. The absorbance at 450 nm was measured at different time points. Data are shown as mean ± SD (n = 3 independent samples). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. (b) Cell growth rate was detected by MTT assay. 14837 T, 14838 T, 8988 T, 8988 S, MIA Paca-2, PL45, HPAC, ASPC, PANC 03.27 and PANC-1 cells were incubated with or without 968 (20 μM). The absorbance at 450 nm was measured at different time points. Data are shown as mean ± SD (n = 3 independent samples). (c, d) Knock-out cell lines of mCAFs or hCAFs were obtained by CRISPR/Cas9 technique. Western blotting was used to detect the knockout efficiency of mCAFs(c) and hCAFs(d). Experiments in c, d were repeated every 2 weeks, with similar results. (e) Cell growth rate was detected by MTT assay. mCAFs and NUFIP1-KO mCAFs cells were incubated with no glutamine media. The absorbance at 450 nm was measured at different time points. Data are shown as mean ± SD (n = 3 biologically independent samples). (f) CRISPR/Cas9-resistent NUFIP1-WT(r) and NUFIP1-W35A(r) were overexpressed in NUFIP1-KO mCAFs. These cells were cultured with or without glutamine for 48 h, and then immunostained with the antibody against NUFIP1 and LC3. The results showed the cellular localization of NUFIP1. Scale bars: 5 μM. Representative of n = 3 independent experiments. Pairwise comparisons were conducted using two-tailed, unpaired Student’s t-tests (b, e).
Extended Data Fig. 2 rRNA degradation is activated in CAFs.
(a, b) Cells were cultured with or without glutamine for 48 h. RNA was analyzed with RT-PCR and normalized to GAPDH. (c) mCAF-2# was cultured with or without glutamine for 48 h. RNA gel showed the degradation of RNA. Representative of n = 3 independent experiments. (d, e) mCAF-1#(d) and mCAF-2# (e) were cultured with or without glutamine for 48 h. RNA was analyzed with RT-PCR and normalized to GAPDH. (f) hCAF-2# was cultured with or without glutamine for 48 h. RNA gel showed the degradation of RNA. Representative of n = 3 independent experiments. (g-i) hCAF-1#(g), hCAF-2# (h) or autophagy-deficient mCAFs (i) were cultured with or without glutamine for 48 h. RNA was analyzed with RT-PCR and normalized to GAPDH. (j, k) Wild-type or autophagy-deficient hCAFs were cultured with or without glutamine for 48 h. RNA gel showed the degradation of RNA (j). Representative of n = 3 independent experiments. RNA was also analyzed with RT-PCR and normalized to GAPDH (k). (l) 14837 T infected with virus of flag-NUFIP1-WT or flag-NUFIP1-W35A was immunoprecipitated using M2 beads. The co-precipitated RNAs were purified and analyzed by RT-qPCR using primers for the indicated RNA species. The relative rRNA level was normalized to the input. Data are shown as mean ± SD (n = 3 independent experiments). Pairwise comparisons were conducted using two-tailed, unpaired Student’s t-tests. (m) 14837 T infected with virus of Flag-NUFIP1-WT and Flag-NUFIP1-W35A for 48 h and cultured with or without glutamine for 48 h. Then cells were immunoprecipitated using M2 beads. The co-precipitation proteins were detected by WB. Representative of n = 3 independent experiments. (n-o) WT mCAFs, NUFIP1-KO mCAFs(n) or autophagy-deficient mCAFs (o) were cultured with or without glutamine for 48 h under actinomycin D treatment. RNA was analyzed with RT-PCR and normalized to GAPDH. Data are shown as mean ± SD (3 times the experiment was repeated with similar results) (a, b, d, e, g-i, k, n, o).
Extended Data Fig. 3 NUFIP1 translocated from the nucleus to the cytoplasm.
(a, b) 8988 T and hCAFs were cultured with or without glutamine for 48 h and then immunostained with the antibody against NUFIP1. The results showed the cellular localization of NUFIP1 in 8988 T and hCAFs. Scale bars: 5 μM. Representative of n = 3 independent experiments. The average fluorescence intensity of NUFIP1 in nucleus and cytoplasm were recorded (b). Data are shown as mean ± SD (n = 50 cells). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. (c, d) hCAFs were treated with no glutamine media for 48 h. These cells were immunostained with the antibody against NUFIP1, LAMP1 or LC3. The results showed the co localization of NUFIP1 with LAMP1 (c) or LC3 (d). The white arrows point to the co localization of the described protein. Scale bars: 5 μM. Representative of n = 3 independent experiments. (e) STX17-KO mCAFs and NUFIP1/STX17 double-KO mCAFs were obtained by CRISPR/Cas9 technique. Western blotting was used to detect the knockout efficiency. Representative of n = 3 independent experiments.
Extended Data Fig. 4 CAFs secrete nucleosides dependent on NUFIP1.
(a, b) Relative content of nucleosides in cells (a) and medium (b). WT hCAFs and NUFIP1-KO hCAFs were cultured with or without glutamine for 48 h. Metabolic analysis of cells was performed by mass spectrometry. Data are shown as mean ± SD (n = 3 biologically independent samples). (c, d) Relative content of nucleosides in cells (c) and medium (d). NUFIP1-KO mCAFs, NUFIP1-WT mCAFs and NUFIP1-W35A mCAFs were cultured with or without glutamine for 48 h. Metabolic analysis of cells was performed by mass spectrometry. Data are shown as mean ± SD (n = 3 biologically independent samples). (e, f) Relative content of nucleosides in cells (e) and medium (f). WT mCAFs and Atg3-KO mCAFs were cultured with or without glutamine for 48 h. Metabolic analysis of cells was performed by mass spectrometry. Data are shown as mean ± SD (n = 3 biologically independent samples). (g) Relative content of AA in cells. WT mCAFs and NUFIP1-KO mCAFs were cultured with or without glutamine for 48 h. Metabolic analysis of cells was performed by mass spectrometry. Data are shown as mean ± SD (n = 3 biologically independent samples). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test (a-g).
Extended Data Fig. 5 CAFs secrete nucleosides that promote PDAC metabolism.
(a) 8988 T cells were cultured in complete media (4 mM glutamine) or conditioned medium (no glutamine) from different cell lines for 48 h. Nucleosides and nucleotides in the cells were analyzed by mass spectrometry. Data are shown as mean ± SD (one representative of three independent experiments with similar results is shown). (b) 8988 T cells were cultured in complete media (4 mM glutamine), no glutamine media with or without UIAGC (0.5 mM each) or conditioned medium (no glutamine) from WT hCAFs and NUFIP1-KO hCAFs for 48 h. Metabolic analysis of cells was performed by mass spectrometry. Data are shown as mean ± SD (one representative of three independent experiments with similar results is shown). (c) 14837 T cells were cultured in complete media (4 mM glutamine), no glutamine media or conditioned medium (no glutamine) from WT mCAFs and Atg3-KO mCAFs for 48 h. Metabolic analysis of cells was performed by mass spectrometry. Data are shown as mean ± SD (one representative of three independent experiments with similar results is shown). (d) Metabolic map of stable isotope tracer experiment in Extended Data Fig. 5e. (e) 14837 T cells were cultured in no glutamine media with or without 13C5-Adenosine (2 mM) or 13C5-Uridine (2 mM) for 48 h. 13C stable isotope labeled the ribose in nucleoside. The metabolites labeled with stable isotope in cells were detected by mass spectrometry. Data are shown as mean ± SD (one representative of three independent experiments with similar results is shown). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. (a-c, e).
Extended Data Fig. 6 Glycolysis and the TCA cycle were slightly affected by nucleosides under low glucose conditions.
(a, b) 14837 T cells were cultured in complete media (25 mM glucose), low glucose media (1 mM glucose) with or without uridine (U, 0.5 mM), inosine (I, 0.5 mM), adenosine (A, 0.5 mM), guanosine (G, 0.5 mM), cytidine (C, 0.5 mM) for 48 h. The cells were counted to calculate the cell proliferation (a). Metabolic analysis of cells was performed by mass spectrometry (b). Data are shown as mean ± SD. (n = 3 biologically independent samples). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test.
Extended Data Fig. 7 The CAF-CM and nucleosides induced glucose consumption genes expression in PDAC.
(a) Diagram of metabolic enzymes in the glycolysis pathway and TCA cycle. (b, c) hPDAC-1# and hPDAC-2# were cultured in complete media (4 mM glutamine) or conditioned medium (no glutamine) from different cell lines for 48 h. RNA was then extracted and analyzed with RT-PCR. Data are shown as mean ± SD (n = 3 independent experiments). (d, e) hPDAC-1# and hPDAC-2# were cultured in complete media (4 mM glutamine), no glutamine media with or without UIAGC (0.5 mM each) for 48 h. RNA was then extracted and analyzed with RT-PCR. Data are shown as mean ± SD (n = 3 independent experiments). (f) 8988 T cells were cultured in 96 well plates with complete media (4 mM glutamine), no glutamine media with UIAGC (0.5 mM each) or conditioned medium (no glutamine) from different cell lines for 48 h. The relative value of glucose uptake was measured by the Glucose Uptake-GloTM Assay kit. Data are shown as mean ± SD (n = 3 biologically independent samples). (g) 8988 T cells were cultured in different media (no glutamine, 25 mM glucose or 5 mM glucose) with or without UIAGC (0.5 mM each) for 48 h. The cells were counted to calculate the cell proliferation. Data are shown as mean ± SD (n = 4 independent samples). (h) 8988 T cells were cultured in conditioned medium (no glutamine, 25 mM glucose or 5 mM glucose) from different cell lines with for 48 h. The cells were counted to calculate the cell proliferation. Data are shown as mean ± SD (n = 4 independent samples). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test (b-h).
Extended Data Fig. 8 CAFs activate glucose consumption genes expression in PDAC dependent on MYC.
(a) mRNA levels of A2AR, A2BR, CD73 and CD39 in 14837 T cells. Data are shown as mean ± SD (n = 3 independent experiments). (b) 14837 T cells were cultured in complete media (4 mM glutamine) or conditioned medium (no glutamine) from different cell lines for 48 h. RNA was extracted and analyzed with RT-PCR. Data are shown as mean ± SD (n = 3 independent experiments). (c-e) Detect the effects of A2AR inhibitor (SCH58261, 1 μM) and CD73 inhibitor (MethADP, 50 μM) on 14837 T. 14837 T cells were treated with SCH58261 (1 μM) or MethADP (50 μM) and cultured in complete media or conditioned medium (no glutamine) from 14837 T or mCAFs for 48 h. The cells were counted (c). Data are shown as mean ± SD (n = 4 independent samples). WB was performed to determine MYC (d, e). Representative of n = 3 independent experiments. (f-g) 8988 T and 8988 T (MYC knocked-down) cells were cultured in complete media or conditioned medium (no glutamine) from different cell lines for 48 h. RNA was then extracted and analyzed with RT-PCR (f). Data are shown as mean ± SD (n = 3 independent experiments). WB was performed to determine MYC (g). Representative of n = 3 independent experiments. (h-i) 8988 T (WT) and 8988 T (MYC knocked-down) cells were cultured in complete media or no glutamine media with or without UIAGC (0.5 mM each) for 48 h. RNA was then extracted and analyzed with RT-PCR (h). Data are shown as mean ± SD (n = 3 independent experiments). WB was performed to determine MYC (i). Representative of n = 3 independent experiments. (j) 14837 T cells were cultured in complete media (4 mM glutamine), no glutamine media or conditioned medium (no glutamine) from WT mCAFs and Atg3-KO mCAFs for 48 h. Western blotting was performed to determine MYC protein levels. Representative of n = 3 independent experiments. Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. (a-c, f, h).
Extended Data Fig. 9 CAFs promote growth of PDAC dependent on MYC.
(a) 14837 T cells were cultured in conditioned medium (no glutamine) from different cell lines for 48 h. RNA was then extracted and analyzed with RT-PCR. Data are shown as mean ± SD (n = 3 independent experiments). (b) 14837 T cells were cultured in complete media (4 mM glutamine) or conditioned medium (no glutamine) from different cell lines for 48 h. RNA was then extracted and analyzed with RT-PCR. Data are shown as mean ± SD (n = 3 independent experiments). (c) 14837 T (WT) and 14837 T (MYC knocked-down) cells were cultured in complete media (4 mM glutamine) or conditioned medium (no glutamine) from 14837 T or mCAFs for 48 h. RNA was then extracted and analyzed with RT-PCR. Data are shown as mean ± SD (n = 3 independent experiments). (d) 8988 T and 8988 T (MYC knocked-down) cells were cultured in no glutamine media with or without uridine (U, 0.5 mM), inosine (I, 0.5 mM), adenosine (A, 0.5 mM), guanosine (G, 0.5 mM), cytidine (C, 0.5 mM) and UIAGC (0.5 mM each) for 48 h. Then cells were counted. Data are shown as mean ± SD (n = 4 independent samples). (e) 8988 T and 8988 T (MYC knocked-down) cells were cultured in conditioned medium (no glutamine) from 8988 T or hCAFs for 48 h. Then cells were counted. Data are shown as mean ± SD (n = 4 independent samples). (f) 8988 T and 8988 T (MYC knocked-down) cells were cultured in no glutamine media with or without UIAGC for 48 h. Then cells were counted. Data are shown as mean ± SD (n = 4 independent samples). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test(a-f).
Extended Data Fig. 10 NUFIP1 does not affect the desmoplastic response in PDAC.
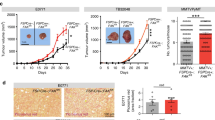
(a-d) 14837 T cells were orthotopically injected into WT, FSP-Cre;ATG5fl/fl and FSP-Cre;NUFIP1fl/fl mice. After 14 days, the tumors were analyzed by Masson trichrome staining (n = 14 views per group) (a, b) and α-SMA immunohistochemical staining (n = 15 views per group) (c, d). Representative images are shown. Scale bars: 20 μM. Data are shown as mean ± SD. Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. (e) Diagram of orthotopic syngeneic graft model in FSP-Cre;ATG5fl/fl and FSP-Cre;NUFIP1fl/fl mice. (f) High expression of NUFIP1 (case34) and low expression of NUFIP1 (case7) in tumor was shown. Scale bars: 10 μM. (g) Kaplan-Meier survival curves for PDAC patients with low (blue) or high (red) tumor expression of NUFIP1, as assessed by IHC. Statistical analysis was performed using Gehan–Breslow–Wilcoxon test; n = 80 patients.
Supplementary information
Supplementary Information
Supplementary Tables 1–4.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, M., Tu, B., Li, H. et al. Cancer-associated fibroblasts employ NUFIP1-dependent autophagy to secrete nucleosides and support pancreatic tumor growth. Nat Cancer 3, 945–960 (2022). https://doi.org/10.1038/s43018-022-00426-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-022-00426-6
This article is cited by
-
Autophagy inhibition improves the targeted radionuclide therapy efficacy of 131I-FAP-2286 in pancreatic cancer xenografts
Journal of Translational Medicine (2024)
-
Targeting stromal metabolism in pancreatic ductal adenocarcinoma
Nature Cell Biology (2024)
-
The Lin28b/Wnt5a axis drives pancreas cancer through crosstalk between cancer associated fibroblasts and tumor epithelium
Nature Communications (2023)
-
Stromal cells in the tumor microenvironment: accomplices of tumor progression?
Cell Death & Disease (2023)
-
GNL3L promotes autophagy via regulating AMPK signaling in esophageal cancer cells
Medical Oncology (2023)