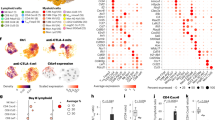
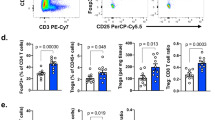
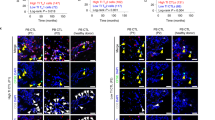
Abstract
Evasion of antitumor immunity and resistance to therapies in solid tumors are aided by an immunosuppressive tumor microenvironment (TME). We found that TME factors, such as regulatory T cells and adenosine, downregulated type I interferon receptor IFNAR1 on CD8+ cytotoxic T lymphocytes (CTLs). These events relied upon poly-ADP ribose polymerase-11 (PARP11), which was induced in intratumoral CTLs and acted as a key regulator of the immunosuppressive TME. Ablation of PARP11 prevented loss of IFNAR1, increased CTL tumoricidal activity and inhibited tumor growth in an IFNAR1-dependent manner. Accordingly, genetic or pharmacologic inactivation of PARP11 augmented the therapeutic benefits of chimeric antigen receptor T cells. Chimeric antigen receptor CTLs engineered to inactivate PARP11 demonstrated a superior efficacy against solid tumors. These findings highlight the role of PARP11 in the immunosuppressive TME and provide a proof of principle for targeting this pathway to optimize immune therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Mouse scRNA-seq data are all available on the Gene Expression Omnibus (accession no. GSE171055). Source data have been provided as Source Data files. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Jhunjhunwala, S., Hammer, C. & Delamarre, L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer. 21, 298–312 (2021).
O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151–167 (2019).
Swann, J. B. & Smyth, M. J. Immune surveillance of tumors. J. Clin. Invest. 117, 1137–1146 (2007).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Liu, C., Workman, C. J. & Vignali, D. A. Targeting regulatory T cells in tumors. FEBS J. 283, 2731–2748 (2016).
Veglia, F. & Gabrilovich, D. I. Dendritic cells in cancer: the role revisited. Curr. Opin. Immunol. 45, 43–51 (2017).
Vijayan, D., Young, A., Teng, M. W. L. & Smyth, M. J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 17, 709–724 (2017).
Allard, B., Allard, D., Buisseret, L. & Stagg, J. The adenosine pathway in immuno-oncology. Nat. Rev. Clin. Oncol. 17, 611–629 (2020).
Smyth, M. J., Ngiow, S. F., Ribas, A. & Teng, M. W. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 13, 143–158 (2016).
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Fuchs, S. Y. Hope and fear for interferon: the receptor-centric outlook on the future of interferon therapy. J. Interferon Cytokine Res. 33, 211–225 (2013).
Parker, B. S., Rautela, J. & Hertzog, P. J. Antitumour actions of interferons: implications for cancer therapy. Nat. Rev. Cancer 16, 131–144 (2016).
Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414 (2015).
Benci, J. L. et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 167, 1540–1554 (2016).
Aichele, P. et al. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J. Immunol. 176, 4525–4529 (2006).
Curtsinger, J. M., Valenzuela, J. O., Agarwal, P., Lins, D. & Mescher, M. F. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 174, 4465–4469 (2005).
Hervas-Stubbs, S. et al. Effects of IFN-α as a signal-3 cytokine on human naive and antigen-experienced CD8(+) T cells. Eur. J. Immunol. 40, 3389–3402 (2010).
Katlinski, K. V. et al. Inactivation of interferon receptor promotes the establishment of immune privileged tumor microenvironment. Cancer Cell 31, 194–207 (2017).
Bhattacharya, S. et al. Anti-tumorigenic effects of Type 1 interferon are subdued by integrated stress responses. Oncogene 32, 4214–4221 (2013).
Bhattacharya, S. et al. Triggering ubiquitination of IFNAR1 protects tissues from inflammatory injury. EMBO Mol. Med. 6, 384–397 (2014).
Ortiz, A. et al. An Interferon-driven oxysterol-based defense against tumor-derived extracellular vesicles. Cancer Cell 35, 33–45 e36 (2019).
Huangfu, W. C. et al. Inflammatory signaling compromises cell responses to interferon-α. Oncogene 31, 161–172 (2012).
Spiegelman, V. S. et al. Wnt/β-catenin signaling induces the expression and activity of βTrCP ubiquitin ligase receptor. Mol. Cell 5, 877–882 (2000).
Guo, T. et al. ADP-ribosyltransferase PARP11 modulates the interferon antiviral response by mono-ADP-ribosylating the ubiquitin E3 ligase β-TrCP. Nat. Microbiol. 4, 1872–1884 (2019).
Bhattacharya, S. et al. Role of p38 protein kinase in the ligand-independent ubiquitination and down-regulation of the IFNAR1 chain of type I interferon receptor. J. Biol. Chem. 286, 22069–22076 (2011).
Gurusamy, D. et al. Multi-phenotype CRISPR-Cas9 screen identifies p38 kinase as a target for adoptive immunotherapies. Cancer Cell 37, 818–833 (2020).
Alicea-Torres, K. et al. Immune suppressive activity of myeloid-derived suppressor cells in cancer requires inactivation of the type I interferon pathway. Nat. Commun. 12, 1717 (2021).
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Kumar, K. G. et al. Site-specific ubiquitination exposes a linear motif to promote interferon-α receptor endocytosis. J. Cell Biol. 179, 935–950 (2007).
Kumar, K. G., Krolewski, J. J. & Fuchs, S. Y. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. J. Biol. Chem. 279, 46614–46620 (2004).
Kumar, K. G. et al. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-α receptor. EMBO J. 22, 5480–5490 (2003).
Li, Y., Gazdoiu, S., Pan, Z. Q. & Fuchs, S. Y. Stability of homologue of Slimb F-box protein is regulated by availability of its substrate. J. Biol. Chem. 279, 11074–11080 (2004).
Meyer-Ficca, M. L. et al. Spermatid head elongation with normal nuclear shaping requires ADP-ribosyltransferase PARP11 (ARTD11) in mice. Biol. Reprod. 92, 80 (2015).
Khan, O. et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature 571, 211–218 (2019).
Yang, R. et al. Distinct epigenetic features of tumor-reactive CD8+ T cells in colorectal cancer patients revealed by genome-wide DNA methylation analysis. Genome Biol. 21, 2 (2019).
Larson, R. C. & Maus, M. V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21, 145–161 (2021).
Kirby, I. T. et al. A potent and selective PARP11 inhibitor suggests coupling between cellular localization and catalytic activity. Cell Chem. Biol. 25, 1547–1553 (2018).
Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 34, 360–394 (2020).
Maj, T. et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol. 18, 1332–1341 (2017).
Kohlmeier, J. E., Cookenham, T., Roberts, A. D., Miller, S. C. & Woodland, D. L. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity 33, 96–105 (2010).
Piehler, J., Thomas, C., Garcia, K. C. & Schreiber, G. Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation. Immunol. Rev. 250, 317–334 (2012).
Anastas, J. N. & Moon, R. T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13, 11–26 (2013).
Liu, J. et al. Virus-induced unfolded protein response attenuates antiviral defenses via phosphorylation-dependent degradation of the type I interferon receptor. Cell Host Microbe 5, 72–83 (2009).
Minn, A. J. & Wherry, E. J. Combination cancer therapies with immune checkpoint blockade: convergence on interferon signaling. Cell 165, 272–275 (2016).
Minn, A. J. Interferons and the immunogenic effects of cancer therapy. Trends Immunol. 36, 725–737 (2015).
Evgin, L. et al. Oncolytic virus-derived type I interferon restricts CAR T cell therapy. Nat. Commun. 11, 3187 (2020).
Peyraud, F. & Italiano, A. Combined PARP inhibition and immune checkpoint therapy in solid tumors. Cancers 12, 1502 (2020).
Cho, C. et al. Cancer-associated fibroblasts downregulate type I interferon receptor to stimulate intratumoral stromagenesis. Oncogene 39, 6129–6137 (2020).
Bhattacharya, S. et al. Inducible priming phosphorylation promotes ligand-independent degradation of the IFNAR1 chain of type I interferon receptor. J. Biol. Chem. 285, 2318–2325 (2010).
Goldman, L. A. et al. Characterization of antihuman IFNAR-1 monoclonal antibodies: epitope localization and functional analysis. J. Interferon Cytokine Res. 19, 15–26 (1999).
Acknowledgements
This work was supported by the by the National Institutes of Health (NIH)/National Cancer Institute R01 grants CA247803 (to S.Y.F. and D.P.B.), R01 CA240814 (to S.Y.F. and A.B.) and P01 CA165997 grant (to J.A.D., C.K. and S.Y.F.), NIH/NICHD grant R15 HD100970 (to R.G.M), NIH/NIA grant R56 AG069745 (to M.M.F.), K08 CA252619 (to M.J.A.) and National Natural Science Foundation of China (31970846 for Hui Z.). We thank staff at the Human Immunology Core at the University of Pennsylvania for providing purified human leukocyte subsets for our research. We are grateful to S. Ostrand-Rosenberg (University of Maryland) and E. Moon (University of Pennsylvania) for providing reagents. We greatly appreciate technical advice from A. Phan, L. Shallberg and A. Rotolo (University of Pennsylvania). We also thank D. Gabrilovich (Astra-Zeneca), A. Gamero (Temple University) and the members of the Fuchs, Minn and Koumenis Ryeom laboratories for critical suggestions.
Author information
Authors and Affiliations
Contributions
S.Y.F., H. Zhang, H. Zheng, M.C.M., A.J.M., J.A.D. and C.K. designed the research. H. Zhang, P.Y., V.S.T., X.C., M.J.A., Z.L., H-G.Z., S.L., A.O., J.G., N.A.L., F.Y., A.B., D.P.B., J.L., S.N.-C., R.S.O., L.R.J. and S.G. directly performed the experiments and interpreted the data. M.L.M-F. and R.G.M. provided key resources and valuable advice. S.Y.F., H. Zhang, H. Zheng, A.J.M., M.J.A., M.C.M., A.B., J.A.D. and C.K. wrote the manuscript with the help of all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Downregulation of IFNAR1 on the intratumoral CTL undermines their activities.
a Growth of MC38 tumors (1 × 106/mouse) after s.c. injection into WT and SA mice. Tumor volumes were measured 3 times per week. Data are shown as mean ± SEM (WT, n = 8 mice; SA, n = 5 mice). Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparisons test. ***P = 0.0004. b t-SNE plots showing the expression of Itgam, Cd3d, Cd8a and Cd4. Transcript levels are color-coded. N = 9,725 cells. c Dot plots showing the expression of CD8+ T cell function relevant genes that are highly expressed in each cluster. The size of the dot corresponds to the percentage of cells expressing the gene in each group and the color represents the average expression level. WT, n = 2075 cells; SA, n = 2038 cells. d Flow cytometry gating strategies in analysis of cellular components of tumor tissues. e Flow cytometry analysis of CD69+ and FasL+ GzmB+ cells gated on CD45+CD3+CD8+ T cells in indicated tumor tissues. Data are shown as mean ± SEM (n = 6 mice for each group). Two-tailed unpaired t-test was performed for the comparisons between groups.
Extended Data Fig. 2 Downregulation of CTL IFNAR1 by Treg and adenosine.
a WT or SA OT-1 cells were first co-cultured with or without iTregs for 24 h (Treg:OT-1 = 1:3) and then combined with MC38OVA-luc cells at indicated E:T ratios. Killing of MC38OVA-luc cells was analyzed using luciferase assay as described in Methods. Data are shown as mean ± SEM (n = 3 co-cultures.) Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparisons test. *P = 0.0288. b Tumor associated Tregs were isolated from MC38 WT tumors and cocultured with OT-1 cells for 24 h (Treg: OT-1 = 1:3). Then analysis of IFNAR1 levels on OT-1 CTL as well as killing of MC38OVA-luc cells (co-cultured at ratio E:T = 10:1) was performed as described in Methods. Data are shown as mean ± SEM (WT and SA IFNAR1, n = 3 co-cultures; WT and SA lysis%, n = 6 co-cultures Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. c Representative flow cytometry analysis of levels of IFNAR1 on the surface of CD3+CD8+ T cells treated with or without adenosine (Ado, 1 mM) prostaglandin E2 (PGE2, 1 μg/ml) or tumor growth factor-beta (TGF-β, 5 ng/ml) for 2 h. d Lysis of MC38OVA-luc cells co-cultured in vitro with OT-1 cells pretreated or not with adenosine (Ado, 1 mM for 24 h) at indicated E:T ratios. Data are shown as mean ± SEM (n = 3 co-cultures). Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparisons test. ****P < 0.0001. e Schematic of crosses to generate the Ifnar1ΔCD8 mice. f Genotyping analysis of Ifnar1ΔCD8 mice by PCR. g Validation of Ifnar1 ablation in CD8+ cells using the flow cytometry analysis of IFNAR1 levels on the surface of CD4+ and CD8+ T cells in Ifnar1+/+ and Ifnar1ΔCD8 mice. It is representative of n = 3 independent experiments.
Extended Data Fig. 3 PARP11 regulates IFNAR1 downregulation in CD8+ T cells.
a qPCR analysis of mRNA of indicated genes in Jurkat cells treated with adenosine (Ado, 1 mM) for 30 min. Data are shown as mean ± SEM (n = 3 independently treated cell cultures.). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. ****P < 0.0001. b Flow cytometry analysis of IFNAR1 levels in EL4 cells, in which indicated genes were knocked out by sgRNA mediated CRISPR-Cas9. Data are shown as mean ± SEM. n = 6 independently treated cell cultures. Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. ****P < 0.0001. c Immunoblot and qPCR analysis of efficiency of indicated genes knockout (due to the lack of PARP11-specific antibodies, levels of expression of PARP11 is demonstrated by showing the product of qPCR). Levels of β-actin and β-tubulin are shown as loading controls. d ADP-ribosylation of HA-β-TrCP immunoprecipitated from 293 T cells treated or not with adenosine (Ado, 1 mM, 30 min) as indicated. After treatment, levels of HA-β-TrCP in the whole-cell lysate (WCL) was analyzed by immunoblotting. Normalized amounts of lysate containing comparable levels of HA-β-TrCP were taken into immunoprecipitation with HA antibody, which was then analyzed by immunoblotting using anti-ADP-ribose antibody and HA antibody. It is representative of 3 independent repeats with similar results. e Representative genotyping of Parp11−/− and Ifnar1−/− mice. e Representative flow cytometry data for Fig. 3g, h. f Representative flow cytometry data for Fig. 3j. g Representative flow cytometry data for Fig. 3k.
Extended Data Fig. 4 Inactivation of intratumoral CTL and robust tumor growth require IFNAR1-dependent function of PARP11 in the TME.
a Flow cytometry analysis of levels of IFNAR1 on the surface of CD45+CD3+CD8+ T cells in tumor tissues from WT, Parp11−/− and Parp11−/−Ifnar1−/− mice 13 days after inoculation of s.c. B16F10 tumors (0.5 × 105/ mouse). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. n = 5 mice. b Growth of s.c. LLC tumors (1 × 106/ mouse) inoculated into WT or Parp11−/− mice. Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparisons test. ****P < 0.0001. WT, n = 6 mice; Parp11−/−, n == 5 mice. c Growth of s.c. B16F10 tumor cells (0.5 × 105/ mouse) inoculated into WT, Parp11−/−, or Parp11−/−Ifnar1−/− mice. Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparisons test. *P = 0.0387, ****P < 0.0001. n = 5 mice. d Quantification of B16F10 tumor weights in mice of indicated genotypes at day 13 after inoculation. Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. ****P < 0.0001. n = 5 mice. e Flow cytometry analysis of numbers of CD8+ T cells in B16F10 tumors growing in indicated mice. Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. ***P = 0.0007. n = 5 mice. f Flow cytometry analysis of IFN-γ+ cells gated on CD45+CD3+CD8+ T cells in B16F10 tumors growing in indicated mice. Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. n = 5 mice. g Flow cytometry analysis of TNF-α+ cells gated on CD45+CD3+CD8+ T cells in B16F10 tumors growing in indicated mice. Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. n = 5 mice. h Flow cytometry analysis of CD69+ cells gated on CD45+CD3+CD8+ T cells in B16F10 tumors growing in indicated mice. Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. n = 5 mice.
Extended Data Fig. 5 PARP11 undermines tumoricidal activities of CAR T cells.
a Proliferation of CD19-BBz CAR T cells prepared from WT or Parp11−/− mouse splenic T cells. Data are shown as mean ± SEM (n = 5 samples for each group). Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparisons test. ns, P = 0.6599. b Flow cytometry analysis of CAR expression in mouse T cells of indicated genotypes after Meso–BBz transduction. c Representative flow cytometry analysis of CAR expression in mouse T cells of indicated genotypes after CD19-BBz transduction. d Weight of hCD19-B16F10 s.c. tumors that grew in NSG mice treated with PBS (Control) or adoptively transferred with CD19-BBz CAR T cells (1 × 106/ mouse, i.v) of indicated genotypes as in Fig. 7d. Data are shown as mean ± SEM (n = 5 mice for each group). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. **P = 0.0087, ****P < 0.0001, *P = 0.0175. e Cell surface expression of human CD19 on the surface of hCD19-B16F10 malignant cells is checked by flow cytometry. Data are shown as mean ± SEM (n = 5 mice for each group). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. f Expression of TIM-3, PD-1 and LAG-3 exhaustion markers by WT or Parp11−/− CAR T cells isolated from hCD19-B16 subcutaneous tumors from mice described in Extended Data Fig. 5d. Data are shown as mean ± SEM (n = 5 mice for each group). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. **P = 0.0270. g Numbers of WT or Parp11−/− CAR T cells in the blood, spleen or hCD19-B16 subcutaneous tumors from mice described in Extended Data Fig. 5d. Data are shown as mean ± SEM (n = 5 mice for each group). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. **P = 0.0038, *P = 0.0212.
Extended Data Fig. 6 Increased efficacy of CAR T cells engineered to inactivate PARP11.
a qPCR analysis of PARP11 mRNA in shCON-CD19-BBz or shPARP11-CD19-BBz CAR T cells. Data are shown as mean ± SEM (n = 5 independently treated cell cultures.). Two-tailed unpaired t-test was performed for the comparisons between groups. ****P < 0.0001. b Flow cytometry analysis of CAR expression in human T cells 3 days after shCON-CD19-BBz or shPARP11-CD19-BBz transduction. c In vitro proliferation of shCON-CD19-BBz and shPARP11-CD19-BBz CAR T cells following stimulation with anti-CD3/CD28 microbeads. Data are shown as mean ± SEM (n = 3 independently treated cell cultures). Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparisons test. d qPCR analysis of PARP11 mRNA in human WT or PARP11 knockout (PARP11 sgRNA) Meso–BBz CAR T cells. Data are shown as mean ± SEM (n = 5 independently treated cell cultures). Two-tailed unpaired t-test was performed for the comparisons between groups. ****P < 0.0001. e Flow cytometry analysis of IFNAR1 cell surface levels on human WT or PARP11 knockout (PARP11 sgRNA) Meso–BBz CAR T cells. Data are shown as mean ± SEM (n = 5 independently treated cell cultures). Two-tailed unpaired t-test was performed for the comparisons between groups. *P = 0.0128. f Analysis of killing of EM-Meso-GFP-Luc cells cocultured with human WT or PARP11 knockout (PARP11 sgRNA) Meso–BBz CAR T cells. Data are shown as mean ± SEM (n = 5 independently treated cell cultures). Two-tailed unpaired t-test was performed for the comparisons between groups. ***P = 0.0006.
Extended Data Fig. 7 Inhibitor of p38 kinase increases the efficacy of CAR T cell therapies.
a Representative flow cytometry analysis of CAR expression in human T cells after Meso–BBz transduction and treatment with vehicle or p38 inhibitor Ralimetinib (LY2228820, LY, 5 μM, 72 h). b Flow cytometry analysis of levels of IFNAR1 on the surface of Meso–BBz CAR T cells pretreated with indicated inhibitors (LY2228820, LY, 5 μM, 72 h) and then treated by adenosine (Ado, 1 mM) for 24 h. Data are shown as mean ± SEM (n = 4 independently treated cell cultures). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. ****P < 0.0001. c Lysis of EM-Meso-GFP-Luc cells by Meso–BBz CAR T pretreated with inhibitors (LY2228820, LY, 5 μM, 72 h) at indicated conditions (E: T = 10:1). Data are shown as mean ± SEM (Veh treated with or without Ado, n = 5 co-cultures; LY treated with or without Ado, n = 5 co-cultures.). Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. **P < 0.0056. d Tumor growth of NSG mice that were injected s.c. with 1 × 106 EM-Meso-GFP-Luc cells. Mice were i.v. treated with 2 × 106 BBZ-SS1 CAR T cells on day 7. BBZ-SS1 CAR T cells were pretreated with LY (5 μM) or Veh for 72 h before injected to mice. Data are shown as mean ± SEM (Control, n = 7 mice; BBZ-SS1, n = 9 mice; BBZ-SS1 + LY, n = 10 mice). Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparisons test. ****P < 0.0001. e The Kaplan-Meier analysis of survival of animals from experiment described in Extended Data Fig. 7d (n = 5 mice for each group). Statistical analysis was performed using Gehan-Breslow-Wilcoxon test.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
About this article
Cite this article
Zhang, H., Yu, P., Tomar, V.S. et al. Targeting PARP11 to avert immunosuppression and improve CAR T therapy in solid tumors. Nat Cancer 3, 808–820 (2022). https://doi.org/10.1038/s43018-022-00383-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-022-00383-0
This article is cited by
-
The ATR inhibitor ceralasertib potentiates cancer checkpoint immunotherapy by regulating the tumor microenvironment
Nature Communications (2024)
-
Tuning CARs: recent advances in modulating chimeric antigen receptor (CAR) T cell activity for improved safety, efficacy, and flexibility
Journal of Translational Medicine (2023)
-
Hypoimmune anti-CD19 chimeric antigen receptor T cells provide lasting tumor control in fully immunocompetent allogeneic humanized mice
Nature Communications (2023)
-
Cancer Stem Cells and the Tumor Microenvironment in Tumor Drug Resistance
Stem Cell Reviews and Reports (2023)
-
Induction of the activating transcription factor-4 in the intratumoral CD8+ T cells sustains their viability and anti-tumor activities
Cancer Immunology, Immunotherapy (2023)