Abstract
Patients with cancer are at higher risk for adverse coronavirus disease 2019 (COVID-19) outcomes. Here, we studied 1,253 patients with cancer, who were diagnosed with severe acute respiratory syndrome coronavirus 2 at a tertiary referral cancer center in India. Most patients had mild disease; in our settings, recent cancer therapies did not impact COVID-19 outcomes. Advancing age, smoking history, concurrent comorbidities and palliative intent of treatment were independently associated with severe COVID-19 or death. Thus, our study provides useful insights into cancer management during the COVID-19 pandemic.
Similar content being viewed by others
Main
The COVID-19 pandemic has infected over 446 million people globally, causing over 6 million deaths (https://covid19.who.int/; accessed 9 March 2022). Global data suggest that increasing age, concurrent illnesses and immunosuppression are risk factors for poor outcomes after COVID-191,2. Patients with cancer are often immunosuppressed by the disease and its treatment; in addition, important predisposing factors for cancer, such as smoking and obesity, also independently contribute to adverse outcomes after COVID-19. Patients with cancer have higher rates of severe disease and fatality after COVID-19 than the general population3. Concurrent comorbidities, poor performance status, specific cancer types and recent systemic anticancer therapy (SACT) have been variably identified as adverse prognostic factors in patients with cancer and COVID-193,4,5. However, the actual risk associated with these factors is unclear.
Forty-three million individuals have been infected with COVID-19 in India, resulting in 515,000 deaths (https://covid19.who.int/, accessed 9 March 2022). Case fatality rates in general have been lower in India compared to other countries, especially Western Europe and the United States. While this has been attributed to underreporting of cases (and deaths), the difference cannot be explained on this basis alone (https://cgdev.org/publication/three-new-estimates-indias-all-cause-excess-mortality-during-covid-19-pandemic, accessed 30 November 2021). Possible factors including acquired immunity due to the population being infected by non-SARSCoV-2 coronaviruses in the past and other less known factors may have contributed to the low fatality rate6. There are scarce data from India on the outcomes of COVID-19 in patients with cancer7,8. Given that cancer treatment is a priority, reliable data are necessary to guide management during future surges of the pandemic. We analyzed the short-term outcomes of COVID-19 in patients with cancer at a tertiary referral cancer center in India and identified risk factors for adverse outcomes.
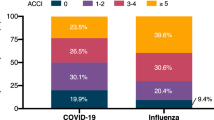
We collected data from 1,253 patients (479 retrospective, from 11 April to 30 June 2020; 774 prospective, from 1 July 2020 to 28 February 2021) with a confirmed diagnosis of cancer and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The patient baseline characteristics are described in Table 1. At a median follow-up of 76 d, 160 patients (12.8%) met the composite end point of severe COVID-19 or death within 30 d of COVID-19 diagnosis. The 30-d all-cause mortality was 10.9% (138 deaths).
The severity of COVID-19 was mild (grade 1–3 on the World Health Organization (WHO) ordinal scale) in 1,014 (81%) patients, moderate (WHO grade 4 or 5) in 167 (13%) patients and severe (WHO grade 6 or 7) in 72 (6%) patients. In patients with limited life expectancy due to advanced cancer who were considered unsuitable for therapy escalation, the severity of COVID-19 was graded according to the treatment provided; therefore, actual severity may have been underestimated. All-cause 30-d mortality was 2.4% (24 out of 1,014), 38.3% (64 out of 167) and 69.4% (50 out of 72), respectively in patients with mild, moderate, and severe COVID-19.
In a multivariable logistic regression analysis for the composite outcome, advancing age, smoking, ≥2 comorbidities, and palliative intent of treatment were independent predictors for worse outcomes (Table 2). A separate multivariable analysis with 30-d mortality as the outcome identified advancing age (odds ratio (OR) = 1.02; 95% confidence interval (CI) = 1.01–1.03; P = 0.003) and palliative intent of treatment (OR = 4.05; 95% CI = 2.73–5.99; P < 0.001) as independent risk factors. Among patients treated with palliative intent, 25% (36 out of 145) of those who received SACT <30 d before COVID-19 had an event, compared to 26% (52 out of 199) in those who did not. In patients older than 65 years who received SACT <30 d before COVID-19 (10 out of 34), 29% experienced the composite end point compared to 20% (25 out of 125) in those who had not received SACT.
In our cohort of patients with cancer who developed COVID-19, advancing age, smoking history, palliative intent of treatment and presence of ≥2 comorbidities were independent risk factors for severe COVID-19 or death within 30 d. Advancing age and palliative intent of treatment remained independently associated with 30-d mortality. Recent SACT, sex and cancer type were not significantly associated with adverse outcomes.
Since the onset of the COVID-19 pandemic, there have been concerns about the outcomes of COVID-19 in patients with cancer. A meta-analysis (26 studies, 23,736 patients) found a pooled all-cause in-hospital mortality rate of 19%, with nearly threefold higher odds of dying than those without cancer3. Early studies from China suggested that patients with cancer and COVID-19 had considerably poorer survival than the general population, with mortality estimates between 20 and 29%9,10). Subsequent studies from other countries reported short-term mortality rates between 10 and 29%, with the UK and other European countries reporting higher fatality rates4,5,11,12,13.
The dissimilarity in results between studies needs to be interpreted keeping in mind that they were done in different settings and with different population characteristics, at various times, corresponding to different phases of the pandemic. Studies early in the pandemic typically reported higher case fatality rates because little was known about the disease and its management. Also, differences in testing strategies between countries imply that in some studies, patients with cancer who were symptomatic with mild disease and potentially favorable outcomes may not have been identified, compared to those with moderate and severe disease, resulting in higher estimated fatality rates. In addition, the outcomes of patients with cancer and COVID-19 need to be compared to outcomes in the general population for that same country. Countries such as Italy and the UK have reported population case fatality rates of 3–5% compared to 1.1% in India. These differences could be partly related to population characteristics, with developed countries having a high proportion of older individuals with comorbidities14. The age pyramid in low- and middle-income countries like India is skewed toward a higher proportion of younger individuals; similarly, a relatively larger proportion of cancers occur at a younger age than in high-income countries. This is reflected in the median age of patients in our study (44 years), which is much lower than reported in other studies (>65 years)5. Similarly, many African countries where the population is predominantly young have reported low COVID-19 fatality rates15. Other associated factors include time trends in the spread of the pandemic, capacity and strategy for testing and the accuracy of reporting deaths14. The low COVID-19 fatality rate in India could also be because of the decreased severity of infection, possibly due to cross-immunity from exposure to other coronaviruses that are endemic in the population6.
Research on COVID-19 in patients with cancer has focused on identifying prognostic factors to aid risk stratification and early recognition of patients likely to have adverse outcomes. In keeping with the published literature, we found that advancing age was an independent risk factor for poor outcomes after COVID-19; within this group, older patients who had received recent SACT had worse outcomes than those who did not5,10,11. Like other studies, we found that concurrent comorbidities and smoking adversely affected COVID-19 severity and outcomes5,13. Our study also showed no impact of sex, cancer type or recent SACT on COVID-19 outcomes. These findings should be interpreted with the understanding that our cohort was different from other studies in some aspects, such as younger median age, spectrum of cancers and less frequent use of monoclonal antibodies and immunotherapy. Broadly, our findings strongly support the continuation of cancer care in most patients during future surges of the pandemic.
Our results showed that treatment with palliative intent was a significant adverse prognostic factor for COVID-19 outcomes, regardless of whether active anticancer treatment had been recently administered. This can be attributed to the debilitation caused by the cancer itself, compounded by the effects of COVID-19. Our study suggests that treatment of patients with advanced metastatic cancers should be guided by the magnitude of benefit based on the nature of the cancer, expected benefits and toxicities with treatment and potential risks of COVID-19-related complications. This is particularly true when healthcare systems are overwhelmed by COVID-19 and resources diverted to palliative chemotherapy would be at the expense of care delivery to those with other diseases, including patients with cancer who are on treatment with curative intent.
A systematic review found that chemotherapy within 30 d before diagnosis of COVID-19 increased the risk of death but not of severe COVID-19 while other therapies (including radiation and immunotherapy) had no such effect16. While this may be explained on the basis of the intense immunosuppression caused by chemotherapy, it needs to be interpreted cautiously. First, many studies have not been able to capture reliable data on the nature and timing of systemic therapy in relation to COVID-19. Second, studies grouped all anticancer therapy, which would dilute the effect of individual treatments. Third, changes in practice during the pandemic may have resulted in only fitter patients receiving intensive chemotherapy, thus confounding the results.
Our study has several strengths and some limitations. To the best of our knowledge, it is one of the largest single-center studies examining the outcomes of COVID-19 in patients with cancer and provides possibly the most robust prospective data available from this part of the world. Second, this was a pragmatic study that included all patients regardless of age, cancer type or COVID-19 severity. Finally, being a referral center for patients with cancer who developed COVID-19, it is likely to be fairly representative of the real world. One possible limitation is that a small proportion of patients who were relatively less symptomatic but did not have facilities for home isolation were admitted to hospital for social rather than medical reasons, potentially skewing the severity scoring of the illness; however, these numbers were low.
The results of our study have important policy-level implications. We have demonstrated that in our setting, most patients with cancer who developed COVID-19 had mild disease and favorable outcomes. Considering that India has a huge burden of COVID-19 and has had multiple pandemic surges, our findings are important to assuage fear in patients and treatment providers. With growing realization of the adverse outcomes of deferring active cancer treatment, our results support continuation of cancer care even during pandemics. Cancer treatment during the pandemic has been severely hampered due to multiple reasons: inability of patients to access care due to fear of contracting COVID-19 or travel restrictions; reduction in existing cancer care facilities because of conversion to COVID-19 centers or staffing issues (illness, quarantine or travel restrictions); and recommendations to downscale or delay cancer therapies. A study across 41 cancer centers in India found substantial reductions in care delivery during the pandemic17. Even in the pre-pandemic period, several low- and middle-income countries faced challenges with cancer care related to lack of access, delayed stage presentation and poor outcomes18. In such settings, further reductions in cancer care are likely to have disastrous consequences. Many countries are now seeing new waves of COVID-19 infections and the findings of this study reinforce that cancer care should be prioritized even during a pandemic.
Methods
We performed an ambi-directional cohort study of patients with cancer diagnosed on PCR with reverse transcription (RT–PCR) with SARS-CoV-2 infection at the Tata Memorial Hospital, Mumbai. The study was approved by the institutional ethics committee, registered with the Clinical Trials Registry of India (CTRI/2020/07/026339) and carried out in accordance with the principles of good clinical research practice.
We included all patients (adult and pediatric) with a proven cancer diagnosis at any stage of management (under evaluation, on active treatment (curative or palliative intent) or on follow-up), with SARS-CoV-2 infection confirmed by a positive RT–PCR test during the study period; these patients were identified from a central database of all patients undergoing RT–PCR testing for suspected SARS-CoV-2 infection.
We collected data from electronic medical records for the following variables: age; sex; comorbidities; smoking status (ever versus never-smoker); date of cancer diagnosis; type of cancer; intent of management (curative versus palliative); status of management (evaluation, active treatment, follow-up); type of management (chemotherapy, radiation therapy, surgery, palliative care, combination or other); date of completion of last systemic anticancer treatment (defined as chemotherapy, immunotherapy, targeted therapy or a combination); date of COVID-19 diagnosis; maximum severity of COVID-19 (classified using the WHO ordinal scale); and COVID-19 outcome (dead or alive). The WHO ordinal scale uses the intervention used to treat COVID-19 as a measure of severity, and not the symptoms; hence, it would underestimate the severity in situations where care was not escalated due to the terminal nature of a comorbid disease such as cancer. Therefore, we used a composite outcome of severe COVID-19 (WHO grade ≥6) or death within 30 d from COVID-19 diagnosis as our primary outcome. We used a multivariable logistic regression model for the association between independent predictors—age, sex, smoking status, presence of comorbidities, cancer type, intent of management, duration from last SACT to COVID-19 diagnosis and primary outcome. We also conducted a multivariable analysis to identify risk factors for 30-d mortality. Data were collected and analyzed with SPSS v.25.0; statistical tests were interpreted at a two-tailed 5% significance level.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
The manuscript has no computer code or algorithms.
References
Booth, A. et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS ONE 16, e0247461 (2021).
Flook, M. et al. Informing the public health response to COVID-19: a systematic review of risk factors for disease, severity, and mortality. BMC Infect. Dis. 21, 342 (2021).
Venkatesulu, B. P. et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. JNCI Cancer Spectr. 5, pkaa102 (2021).
Robilotti, E. V. et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 26, 1218–1223 (2020).
Kuderer, N. M. et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 395, 1907–1918 (2020).
Chakrabarti, S. S. et al. COVID-19 in India: are biological and environmental factors helping to stem the incidence and severity? Aging Dis. 11, 480–488 (2020).
Ramaswamy, A. et al. COVID-19 in cancer patients on active systemic therapy. Outcomes from LMIC scenario with an emphasis on need for active treatment. Cancer Med. 9, 8747–8753 (2020).
Mehta, A. et al. COVID-19 mortality in cancer patients: a report from a tertiary cancer centre in India. PeerJ. 9, e10599 (2021).
Tian, J. et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 21, 893–903 (2020).
Meng, Y. et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis. J. Hematol. Oncol. 13, 75 (2020).
Lee, L. Y. et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 395, 1919–1926 (2020).
Pinato, D. J. et al. Presenting features and early mortality from SARS-CoV-2 infection in cancer patients during the initial stage of the COVID-19 pandemic in Europe. Cancers (Basel) 12, 1841 (2020).
Lièvre, A. et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19). Eur. J. Cancer 141, 62–81 (2020).
Sorci, G., Faivre, B. & Morand, S. Explaining among-country variation in COVID-19 case fatality rate. Sci. Rep. 10, 18909 (2020).
Lawal, Y. Africa’s low COVID-19 mortality rate: a paradox? Int. J. Infect. Dis. 102, 118–122 (2021).
Yekedüz, E., Utkan, G. & Ürün, Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur. J. Cancer 141, 92–104 (2020).
Ranganathan, P. et al. Impact of COVID-19 on cancer care in India: a cohort study. Lancet Oncol. 22, 970–976 (2021).
Pramesh, C. S. et al. Delivery of affordable and equitable cancer care in India. Lancet Oncol. 15, e223–e233 (2014).
Acknowledgements
We acknowledge the clinical support and help with data collection provided by the TMH COVID-19 action group. The authors received no specific funding for this work.
Author information
Authors and Affiliations
Consortia
Contributions
M.S., G.C., P.R. and C.S.P. conceptualized and designed the study, analyzed the data and co-wrote the paper. M.S. and G.C. contributed equally to all aspects of the paper and should be considered joint first authors. A.A., S.B., P.C., C.D., J.D., K.D’S., H.J., S.L., N.R.M., N.M., S.N., L.N., P.N. and S.P. contributed to data collection, analyses and interpretation, revised the paper and approved the final draft. P.P., A.R., O.S., A.S., E.S., J.T. and R.B. contributed to data collection, review of manuscript and approval of final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Justin Gainor, Samuel Rubinstein and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sengar, M., Chinnaswamy, G., Ranganathan, P. et al. Outcomes of COVID-19 and risk factors in patients with cancer. Nat Cancer 3, 547–551 (2022). https://doi.org/10.1038/s43018-022-00363-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-022-00363-4