Abstract
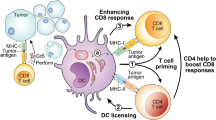
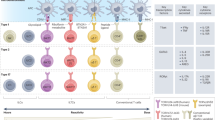
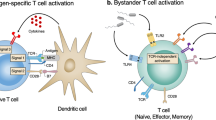
Cancer-specific T cells are required for effective anti-cancer immunity and have a central role in cancer immunotherapy. However, emerging evidence suggests that only a small fraction of tumor-infiltrating T cells are cancer specific, and T cells that recognize cancer-unrelated antigens (so-called ‘bystanders’) are abundant. Although the role of cancer-specific T cells in anti-cancer immunity has been well established, the implications of bystander T cells in tumors are only beginning to be understood. It is becoming increasingly clear that bystander T cells are not a homogeneous group of cells but, instead, they differ in their specificities, their activation states and effector functions. In this Perspective, we discuss recent studies of bystander T cells in tumors, including experimental and computational approaches that enable their identification and functional analysis and viewpoints on how these insights could be used to develop new therapeutic approaches for cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed for this study.
References
Tran, E., Robbins, P. F. & Rosenberg, S. A. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat. Immunol. 18, 255–262 (2017).
Lennerz, V. et al. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc. Natl Acad. Sci. USA 102, 16013–16018 (2005).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Hollingsworth, R. E. & Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 4, 7 (2019).
Gubin, M. M. et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515, 577–581 (2014).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
Samstein, R. M. et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 51, 202–206 (2019).
Simoni, Y. et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018).
Rosato, P. C. et al. Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat. Commun. 10, 567 (2019).
Farah, M. et al. T cell repertoire in combination with T cell density predicts clinical outcomes in patients with Merkel cell carcinoma. J. Invest. Dermatol. 140, 2146–2156 (2020).
Reuben, A. et al. Comprehensive T cell repertoire characterization of non-small cell lung cancer. Nat. Commun. 11, 603 (2020).
Wu, T. D. et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature 579, 274–278 (2020).
Andersen, R. S. et al. Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 72, 1642–1650 (2012).
Scheper, W. et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med. 25, 89–94 (2019).
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018).
Kvistborg, P. et al. TIL therapy broadens the tumor-reactive CD8+ T cell compartment in melanoma patients. OncoImmunology 1, 409–418 (2012).
Tripp, R. A., Hou, S., McMickle, A., Houston, J. & Doherty, P. C. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J. Immunol. 154, 6013–6021 (1995).
Maurice, N. J., Taber, A. K. & Prlic, M. The ugly duckling turned to swan: a change in perception of bystander-activated memory CD8 T cells. J. Immunol. 206, 455–462 (2021).
Chu, T. et al. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep. 3, 701–708 (2013).
Soudja, S. M., Ruiz, A. L., Marie, J. C. & Lauvau, G. Inflammatory monocytes activate memory CD8+ T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 37, 549–562 (2012).
Liu, Z. et al. Detecting tumor antigen-specific T cells via interaction-dependent fucosyl-biotinylation. Cell 183, 1117–1133 (2020).
Tough, D. F., Borrow, P. & Sprent, J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272, 1947–1950 (1996).
Butz, E. A. & Bevan, M. J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8, 167–175 (1998).
Yoshimoto, T. et al. IL-12 up-regulates IL-18 receptor expression on T cells, TH1 cells, and B cells: synergism with IL-18 for IFN-γ production. J. Immunol. 161, 3400–3407 (1998).
Kim, J. et al. Innate-like cytotoxic function of bystander-activated CD8+ T cells is associated with liver injury in acute hepatitis A. Immunity 48, 161–173 (2018).
Freeman, B. E., Hammarlund, E., Raué, H.-P. & Slifka, M. K. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc. Natl Acad. Sci. USA 109, 9971–9976 (2012).
Li, S. et al. Bystander CD4+ T cells infiltrate human tumors and are phenotypically distinct. OncoImmunology 11, 012961 (2022).
Christophersen, A. Peptide–MHC class I and class II tetramers: from flow to mass cytometry. HLA 95, 169–178 (2020).
Vigneron, N. Human tumor antigens and cancer immunotherapy. BioMed Res. Int. 2015, 948501 (2015).
Newell, E. W. et al. Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nat. Biotechnol. 31, 623–629 (2013).
Bentzen, A. K. et al. Large-scale detection of antigen-specific T cells using peptide–MHC-I multimers labeled with DNA barcodes. Nat. Biotechnol. 34, 1037–1045 (2016).
Zhang, S.-Q. et al. High-throughput determination of the antigen specificities of T cell receptors in single cells. Nat. Biotechnol. 36, 1156–1159 (2018).
Li, H. et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell 176, 775–789 (2018).
Thommen, D. S. & Schumacher, T. N. T cell dysfunction in cancer. Cancer Cell 33, 547–562 (2018).
Pasqual, G. et al. Monitoring T cell–dendritic cell interactions in vivo by intercellular enzymatic labelling. Nature 553, 496–500 (2018).
Morsut, L. et al. Engineering customized cell sensing and response behaviors using synthetic Notch receptors. Cell 164, 780–791 (2016).
Chiou, S.-H. et al. Global analysis of shared T cell specificities in human non-small cell lung cancer enables HLA inference and antigen discovery. Immunity 54, 586–602 (2021).
Pasetto, A. et al. Tumor- and neoantigen-reactive T-cell receptors can be identified based on their frequency in fresh tumor. Cancer Immunol. Res. 4, 734–743 (2016).
Rossjohn, J. et al. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 33, 169–200 (2014).
Bagaev, D. V. et al. VDJdb in 2019: database extension, new analysis infrastructure and a T-cell receptor motif compendium. Nucleic Acids Res. 48, D1057–D1062 (2019).
Tickotsky, N., Sagiv, T., Prilusky, J., Shifrut, E. & Friedman, N. McPAS-TCR: a manually curated catalogue of pathology-associated T cell receptor sequences. Bioinformatics 33, 2924–2929 (2017).
Huang, H., Wang, C., Rubelt, F., Scriba, T. J. & Davis, M. M. Analyzing the Mycobacterium tuberculosis immune response by T-cell receptor clustering with GLIPH2 and genome-wide antigen screening. Nat. Biotechnol. 38, 1194–1202 (2020).
Glanville, J. et al. Identifying specificity groups in the T cell receptor repertoire. Nature 547, 94–98 (2017).
Dash, P. et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature 547, 89–93 (2017).
Sidhom, J.-W., Larman, H. B., Pardoll, D. M. & Baras, A. S. DeepTCR is a deep learning framework for revealing sequence concepts within T-cell repertoires. Nat. Commun. 12, 1605 (2021).
Montemurro, A. et al. NetTCR-2.0 enables accurate prediction of TCR–peptide binding by using paired TCRα and β sequence data. Commun. Biol. 4, 1060 (2021).
Jokinen, E., Huuhtanen, J., Mustjoki, S., Heinonen, M. & Lähdesmäki, H. Predicting recognition between T cell receptors and epitopes with TCRGP. PLoS Comput. Biol. 17, e1008814 (2021).
Mognol, G. P. et al. Exhaustion-associated regulatory regions in CD8+ tumor-infiltrating T cells. Proc. Natl Acad. Sci. USA 114, E2776–E2785 (2017).
Joncker, N. T. et al. Antigen‐independent accumulation of activated effector/memory T lymphocytes into human and murine tumors. Int. J. Cancer 118, 1205–1214 (2006).
Thompson, E. D., Enriquez, H. L., Fu, Y.-X. & Engelhard, V. H. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J. Exp. Med. 207, 1791–1804 (2010).
Erkes, D. A. et al. Virus-specific CD8+ T cells infiltrate melanoma lesions and retain function independently of PD-1 expression. J. Immunol. 198, 2979–2988 (2017).
Dangaj, D. et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell 35, 885–900 (2019).
Lanitis, E., Dangaj, D., Irving, M. & Coukos, G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann. Oncol. 28, xii18–xii32 (2017).
Vilgelm, A. E. & Richmond, A. Chemokines modulate immune surveillance in tumorigenesis, metastasis, and response to immunotherapy. Front. Immunol. 10, 333 (2019).
Maurice, N. J., McElrath, M. J., Andersen-Nissen, E., Frahm, N. & Prlic, M. CXCR3 enables recruitment and site-specific bystander activation of memory CD8+ T cells. Nat. Commun. 10, 4987 (2019).
Cheng, Y. et al. Non-terminally exhausted tumor-resident memory HBV-specific T cell responses correlate with relapse-free survival in hepatocellular carcinoma. Immunity 54, 1825–1840 (2021).
Lippitz, B. E. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 14, e218–e228 (2013).
Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414 (2015).
Danahy, D. B., Berton, R. R. & Badovinac, V. P. Cutting edge: antitumor immunity by pathogen-specific CD8 T cells in the absence of cognate antigen recognition. J. Immunol. 204, 1431–1435 (2020).
Caushi, J. X. et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature 596, 126–132 (2021).
Oliveira, G. et al. Phenotype, specificity and avidity of antitumour CD8+ T cells in melanoma. Nature 596, 119–125 (2021).
Yost, K. E. et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25, 1251–1259 (2019).
Krishna, C. et al. Single-cell sequencing links multiregional immune landscapes and tissue-resident T cells in ccRCC to tumor topology and therapy efficacy. Cancer Cell 39, 662–677 (2021).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Utzschneider, D. T. et al. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427 (2016).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Siddiqui, I. et al. Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211 (2019).
Schietinger, A. et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 45, 389–401 (2016).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Leun, A. M., van der, Thommen, D. S. & Schumacher, T. N. CD8+ T cell states in human cancer: insights from single-cell analysis. Nat. Rev. Cancer 20, 218–232 (2020).
Sewell, A. K. Why must T cells be cross-reactive? Nat. Rev. Immunol. 12, 669–677 (2012).
Birnbaum, M. E. et al. Deconstructing the peptide–MHC specificity of T cell recognition. Cell 157, 1073–1087 (2014).
Richman, L. P., Vonderheide, R. H. & Rech, A. J. Neoantigen dissimilarity to the self-proteome predicts immunogenicity and response to immune checkpoint blockade. Cell Syst. 9, 375–382 (2019).
Loftus, D. J. et al. Identification of epitope mimics recognized by CTL reactive to the melanoma/melanocyte-derived peptide MART-1(27–35). J. Exp. Med. 184, 647–657 (1996).
Vujanovic, L., Shi, J., Kirkwood, J. M., Storkus, W. J. & Butterfield, L. H. Molecular mimicry of MAGE-A6 and Mycoplasma penetrans HF-2 epitopes in the induction of antitumor CD8+ T-cell responses. Oncoimmunology 3, e954501 (2014).
Bessell, C. A. et al. Commensal bacteria stimulate antitumor responses via T cell cross-reactivity. JCI Insight 5, e135597 (2020).
Balachandran, V. P. et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512–516 (2017).
Wells, D. K. et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell 183, 818–834 (2020).
Newman, J. H. et al. Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an immunotherapy for cancer. Proc. Natl Acad. Sci. USA 117, 1119–1128 (2020).
McCarthy, E. F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 26, 154–158 (2006).
Simoni, Y. et al. Partial absence of PD‐1 expression by tumor‐infiltrating EBV‐specific CD8+ T cells in EBV‐driven lymphoepithelioma‐like carcinoma. Clin. Transl. Immunol. 9, e1175 (2020).
Iyer, J. G. et al. Merkel cell polyomavirus-specific CD8+ and CD4+ T-cell responses identified in Merkel cell carcinomas and blood. Clin. Cancer Res. 17, 6671–6680 (2011).
Kalaora, S. et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 592, 138–143 (2021).
Cobbs, C. S. et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 62, 3347–3350 (2002).
Taubenberger, J. K. & Morens, D. M. The pathology of influenza virus infections. Annu. Rev. Pathol. 3, 499–522 (2008).
Schuldt, N. J. & Binstadt, B. A. Dual TCR T cells: identity crisis or multitaskers? J. Immunol. 202, 637–644 (2019).
Padovan, E. et al. Expression of two T cell receptor α chains: dual receptor T cells. Science 262, 422–424 (1993).
Sonntag, K. et al. Immune monitoring and TCR sequencing of CD4 T cells in a long term responsive patient with metastasized pancreatic ductal carcinoma treated with individualized, neoepitope-derived multipeptide vaccines: a case report. J. Transl. Med. 16, 23 (2018).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Yeong, J. et al. Intra-tumoral CD39+CD8+ T cells predict response to PD-1/PD-L1 blockade in patients with NSCLC. J. Thorac. Oncol. 16, 1349–1358 (2021).
Pievani, A. et al. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood 118, 3301–3310 (2011).
Zhang, Y. & Schmidt‐Wolf, I. G. H. Ten‐year update of the international registry on cytokine‐induced killer cells in cancer immunotherapy. J. Cell. Physiol. 235, 9291–9303 (2020).
Tietze, J. K. et al. Delineation of antigen-specific and antigen-nonspecific CD8+ memory T-cell responses after cytokine-based cancer immunotherapy. Blood 119, 3073–3083 (2012).
Wong, H. C., Jeng, E. K. & Rhode, P. R. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8 + T cells into innate-like effector cells with antitumor activity. Oncoimmunology 2, e26442 (2013).
Hu, J. et al. CD8+T cell-specific induction of NKG2D receptor by doxorubicin plus interleukin-12 and its contribution to CD8+T cell accumulation in tumors. Mol. Cancer 13, 34 (2014).
Wrangle, J. M. et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 19, 694–704 (2018).
Batich, K. A. et al. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin. Cancer Res. 23, 1898–1909 (2017).
Sefrin, J. P. et al. Sensitization of tumors for attack by virus-specific CD8+ T-cells through antibody-mediated delivery of immunogenic T-cell epitopes. Front. Immunol. 10, 1962 (2019).
Millar, D. G. et al. Antibody-mediated delivery of viral epitopes to tumors harnesses CMV-specific T cells for cancer therapy. Nat. Biotechnol. 38, 420–425 (2020).
Diaz, R. M. et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 67, 2840–2848 (2007).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 14, 1264–1270 (2008).
Ahmed, N. et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 3, 1094–1101 (2017).
Trabolsi, A., Arumov, A. & Schatz, J. H. T cell-activating bispecific antibodies in cancer therapy. J. Immunol. 203, 585–592 (2019).
Mittal, P. et al. Tumor-unrelated CD4 T cell help augments CD134 plus CD137 dual costimulation tumor therapy. J. Immunol. 195, 5816–5826 (2015).
Garrido, F., Aptsiauri, N., Doorduijn, E. M., Lora, A. M. G. & Hall, Tvan The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 39, 44–51 (2016).
Altman, J. D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996).
Andersen, R. S. et al. Parallel detection of antigen-specific T cell responses by combinatorial encoding of MHC multimers. Nat. Protoc. 7, 891–902 (2012).
Dileepan, T. et al. MHC class II tetramers engineered for enhanced binding to CD4 improve detection of antigen-specific T cells. Nat. Biotechnol. 39, 943–948 (2021).
Schmidt, J. et al. Reversible major histocompatibility complex I–peptide multimers containing Ni2+-nitrilotriacetic acid peptides and histidine tags improve analysis and sorting of CD8+ T cells. J. Biol. Chem. 286, 41723–41735 (2011).
Canale, F. P. et al. CD39 expression defines cell exhaustion in tumor-infiltrating CD8+ T cells. Cancer Res. 78, 115–128 (2017).
Mueller, S. N. & Mackay, L. K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89 (2016).
Jansen, C. S. et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019).
Riley, J. L. PD‐1 signaling in primary T cells. Immunol. Rev. 229, 114–125 (2009).
Ahmadzadeh, M. et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009).
Gros, A. et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 124, 2246–2259 (2014).
Inozume, T. et al. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J. Immunother. 33, 956–964 (2010).
Malek, T. R. & Castro, I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 33, 153–165 (2010).
Raulet, D. H. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 3, 781–790 (2003).
Prajapati, K., Perez, C., Rojas, L. B. P., Burke, B. & Guevara-Patino, J. A. Functions of NKG2D in CD8+ T cells: an opportunity for immunotherapy. Cell. Mol. Immunol. 15, 470–479 (2018).
Watts, T. H. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23, 23–68 (2005).
Ye, Q. et al. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin. Cancer Res. 20, 44–55 (2014).
Acknowledgements
A.T.S. was supported by the National Institutes of Health grant K08CA230188, a Technology Impact Award from the Cancer Research Institute and a Career Award for Medical Scientists from the Burroughs Wellcome Fund. A.T.S. and S.L.M. were supported by the Parker Institute for Cancer Immunotherapy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A.T.S. is a scientific cofounder of Immunai and Cartography Biosciences and receives research funding from Merck Research Laboratories and Allogene Therapeutics. D.K.W. is a scientific cofounder and employee of Immunai and has received consulting fees from Rubius Therapeutics, Illumina and DeepMind. S.L.M. has no conflicts of interest to declare.
Peer review
Peer review information
Nature Cancer thanks George Coukos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meier, S.L., Satpathy, A.T. & Wells, D.K. Bystander T cells in cancer immunology and therapy. Nat Cancer 3, 143–155 (2022). https://doi.org/10.1038/s43018-022-00335-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-022-00335-8
This article is cited by
-
Characterization of tumoricidal activities mediated by a novel immune cell regimen composing interferon-producing killer dendritic cells and tumor-specific cytotoxic T lymphocytes
BMC Cancer (2024)
-
Clinical and translational attributes of immune-related adverse events
Nature Cancer (2024)
-
Personalized neoantigen vaccine enhances the therapeutic efficacy of bevacizumab and anti-PD-1 antibody in advanced non-small cell lung cancer
Cancer Immunology, Immunotherapy (2024)
-
An oncolytic virus delivering tumor-irrelevant bystander T cell epitopes induces anti-tumor immunity and potentiates cancer immunotherapy
Nature Cancer (2024)
-
Gamma delta T-cell-based immune checkpoint therapy: attractive candidate for antitumor treatment
Molecular Cancer (2023)