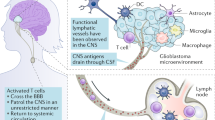
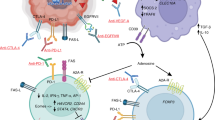
Abstract
Pediatric central nervous system tumors are the most common solid malignancies in childhood, and aggressive therapy often leads to long-term sequelae in survivors, making these tumors challenging to treat. Immunotherapy has revolutionized prospects for many cancer types in adults, but the intrinsic complexity of treating pediatric patients and the scarcity of clinical studies of children to inform effective approaches have hampered the development of effective immunotherapies in pediatric settings. Here, we review recent advances and ongoing challenges in pediatric brain cancer immunotherapy, as well as considerations for efficient clinical translation of efficacious immunotherapies into pediatric settings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
No pertinent data sources were used outside of published literature.
References
Pollack, I. F. Brain tumors in children. N. Engl. J. Med. 331, 1500–1507 (1994).
Cohen, K. J. et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol. 13, 410–416 (2011).
Chan, T. A., Wolchok, J. D. & Snyder, A. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 373, 1984 (2015).
Hamid, O. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369, 134–144 (2013).
Okada, H. et al. Immunotherapeutic approaches for glioma. Crit. Rev. Immunol. 29, 1–42 (2009).
Walker, P. R., Calzascia, T., de Tribolet, N. & Dietrich, P. Y. T-cell immune responses in the brain and their relevance for cerebral malignancies. Brain Res. Brain Res. Rev. 42, 97–122 (2003).
Platten, M. & Reardon, D. A. Concepts for immunotherapies in gliomas. Semin. Neurol. 38, 62–72 (2018).
Engelhardt, B. Molecular mechanisms involved in T cell migration across the blood–brain barrier. J. Neural Transm. 113, 477–485 (2006).
Krakowski, M. L. & Owens, T. The central nervous system environment controls effector CD4+ T cell cytokine profile in experimental allergic encephalomyelitis. Eur. J. Immunol. 27, 2840–2847 (1997).
Albert, M. L. et al. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat. Med. 4, 1321–1324 (1998).
Okada, H. & Pollack, I. F. Cytokine gene therapy for malignant glioma. Expert Opin. Biol. Ther. 4, 1609–1620 (2004).
Okada, H. et al. Gene therapy of malignant gliomas: a pilot study of vaccination with irradiated autologous glioma and dendritic cells admixed with IL-4 transduced fibroblasts to elicit an immune response. Hum. Gene Ther. 12, 575–595 (2001).
Okada, H. et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J. Transl. Med. 5, 67 (2007).
Liau, L. M. et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin. Cancer Res. 11, 5515–5525 (2005).
Wheeler, C. J. et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 68, 5955–5964 (2008).
Yamanaka, R. et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin. Cancer Res. 11, 4160–4167 (2005).
Yu, J. S. et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 64, 4973–4979 (2004).
Liau, L. M. et al. First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 16, 142 (2018).
Dutoit, V. et al. Antigenic expression and spontaneous immune responses support the use of a selected peptide set from the IMA950 glioblastoma vaccine for immunotherapy of grade II and III glioma. Oncoimmunology 7, e1391972 (2018).
Okada, H. et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic–polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 29, 330–336 (2011).
Weller, M. et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 18, 1373–1385 (2017).
Hilf, N. et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 565, 240–245 (2019).
Tocagen. Tocagen Reports Results of Toca 5 Phase 3 Trial in Recurrent Brain Cancer http://ir.tocagen.com/news-releases/news-release-details/tocagen-reports-results-toca-5-phase-3-trial-recurrent-brain (2019).
Filley, A. C., Henriquez, M. & Dey, M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget 8, 91779–91794 (2017).
Bristol Myers Squibb. Bristol-Myers Squibb Announces Phase 3 CheckMate -498 Study Did Not Meet Primary Endpoint of Overall Survival with Opdivo (Nivolumab) Plus Radiation in Patients with Newly Diagnosed MGMT-Unmethylated Glioblastoma Multiforme https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-phase-3-checkmate-498-study-did (2019).
Bristol Myers Squibb. Bristol-Myers Squibb Provides Update on Phase 3 Opdivo (Nivolumab) CheckMate -548 Trial in Patients with Newly Diagnosed MGMT-Methylated Glioblastoma Multiforme https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-provides-update-phase-3-opdivo-nivolumab- (2019).
Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 375, 2561–2569 (2016).
Louis, D. N. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 131, 803–820 (2016).
Sturm, D. et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat. Rev. Cancer 14, 92–107 (2014).
Watanabe, K. et al. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin. Cancer Res. 3, 523–530 (1997).
Reilly, K. M., Loisel, D. A., Bronson, R. T., McLaughlin, M. E. & Jacks, T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat. Genet. 26, 109–113 (2000).
Mackay, A. et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32, 520–537 (2017).
Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482, 226–231 (2012).
Guerreiro Stucklin, A. S. et al. Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat. Commun. 10, 4343 (2019).
Chheda, Z. S. et al. Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J. Exp. Med. 215, 141–157 (2018).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Sonabend, A. M. et al. Medulloblasoma: challenges for effective immunotherapy. J. Neurooncol. 108, 1–10 (2012).
Rutledge, W. C. et al. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin. Cancer Res. 19, 4951–4960 (2013).
Krishnadas, D. K., Bai, F. & Lucas, K. G. Targeting cancer-testis antigens in recurrent pediatric brain tumors. J. Neurooncol. 123, 193–195 (2015).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Wainwright, D. A. et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin. Cancer Res. 18, 6110–6121 (2012).
Ke, X. et al. Roles of CD4+CD25high FOXP3+ Tregs in lymphomas and tumors are complex. Front. Biosci. 13, 3986–4001 (2008).
Lohr, J. et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin. Cancer Res. 17, 4296–4308 (2011).
Duraiswamy, J., Kaluza, K. M., Freeman, G. J. & Coukos, G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 73, 3591–3603 (2013).
Kodumudi, K. N., Weber, A., Sarnaik, A. A. & Pilon-Thomas, S. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J. Immunol. 189, 5147–5154 (2012).
Margol, A. et al. Tumor associated macrophages in SHH subgroup of medulloblastomas. Clin. Cancer Res. 21, 1457–1465 (2014).
Askew, K. et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 18, 391–405 (2017).
Heimberger, A. B. et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin. Cancer Res. 14, 5166–5172 (2008).
Bouffet, E. et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J. Clin. Oncol. 34, 2206–2211 (2016).
Schlager, C. et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530, 349–353 (2016).
Beatty, G. L. & Moon, E. K. Chimeric antigen receptor T cells are vulnerable to immunosuppressive mechanisms present within the tumor microenvironment. Oncoimmunology 3, e970027 (2014).
van der Burg, S. H., Arens, R., Ossendorp, F., van Hall, T. & Melief, C. J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 16, 219–233 (2016).
Ochs, K. et al. K27M-mutant histone-3 as a novel target for glioma immunotherapy. Oncoimmunology. 6, e1328340 (2017).
Gattinoni, L. et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 202, 907–912 (2005).
Suryadevara, C. M. et al. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology 7, e1434464 (2018).
Desjardins, A. et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 379, 150–161 (2018).
Fried, I. et al. Preliminary results of immune modulating antibody MDV9300 (pidilizumab) treatment in children with diffuse intrinsic pontine glioma. J. Neurooncol. 136, 189–195 (2018).
Geoerger, B. Anti-PD-1 shows promise against advanced paediatric Hodgkin lymphoma—author’s reply. Lancet Oncol. 21, e127 (2020).
Kostine, M. et al. Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur. J. Cancer 157, 474–484 (2021).
Grabovska, Y. et al. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat. Commun. 11, 4324 (2020).
Cloughesy, T. F. et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 25, 477–486 (2019).
Gholamin, S. et al. Disrupting the CD47–SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl. Med. 9, eaaf2968 (2017).
Choi, B. D. et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc. Natl Acad. Sci. USA 110, 270–275 (2013).
Choi, B. D. et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 37, 1049–1058 (2019).
Heiss, J. D. et al. Phase I trial of convection-enhanced delivery of IL13–Pseudomonas toxin in children with diffuse intrinsic pontine glioma. J. Neurosurg. Pediatr. 23, 333–342 (2018).
Souweidane, M. M. et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 19, 1040–1050 (2018).
Yerrabelli, R. S. et al. IntraOmmaya compartmental radioimmunotherapy using 131I-omburtamab-pharmacokinetic modeling to optimize therapeutic index. Eur. J. Nucl. Med. Mol. Imaging 48, 1166–1177 (2021).
He, P. et al. Two-compartment model of radioimmunotherapy delivered through cerebrospinal fluid. Eur. J. Nucl. Med. Mol. Imaging 38, 334–342 (2011).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Hong, J. J. et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin. Cancer Res. 16, 4892–4898 (2010).
Goff, S. L. et al. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. J. Immunother. 42, 126–135 (2019).
Ahmed, N. et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 3, 1094–1101 (2017).
Akhavan, D. et al. CAR T cells for brain tumors: lessons learned and road ahead. Immunol. Rev. 290, 60–84 (2019).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
Mount, C. W. et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M. Nat. Med. 24, 572–579 (2018).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Majzner, R. G. et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin. Cancer Res. 25, 2560–2574 (2019).
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
Patterson, J. D., Henson, J. C., Breese, R. O., Bielamowicz, K. J. & Rodriguez, A. CAR T cell therapy for pediatric brain tumors. Front. Oncol. 10, 1582 (2020).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Khuong-Quang, D. A. et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 124, 439–447 (2012).
Mueller, S. et al. Mass cytometry detects H3.3K27M-specific vaccine responses in diffuse midline glioma. J. Clin. Invest. 130, 6325–6337 (2020).
Pollack, I. F. et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic–polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J. Clin. Oncol. 32, 2050–2058 (2014).
Pollack, I. F. et al. Antigen-specific immunoreactivity and clinical outcome following vaccination with glioma-associated antigen peptides in children with recurrent high-grade gliomas: results of a pilot study. J. Neurooncol. 130, 517–527 (2016).
Ahluwalia, M. et al. SurVaxM with standard therapy in newly diagnosed glioblastoma: phase II trial update. J. Clin. Oncol. 37, 15_suppl (2016).
Fenstermaker, R. A. et al. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol. Immunother. 65, 1339–1352 (2016).
Flores, C. et al. Massive clonal expansion of medulloblastoma-specific T cells during adoptive cellular therapy. Sci. Adv. 5, eaav9879 (2019).
Aurelian, L. Oncolytic viruses as immunotherapy: progress and remaining challenges. Onco Targets Ther. 9, 2627–2637 (2016).
Regina, A. et al. ANG4043, a novel brain-penetrant peptide–mAb conjugate, is efficacious against HER2-positive intracranial tumors in mice. Mol. Cancer Ther. 14, 129–140 (2015).
Haanen, J. B. A. G. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv264–iv266 (2018).
Brahmer, J. R., Lacchetti, C. & Thompson, J. A. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline summary. J. Oncol. Pract. 14, 247–249 (2018).
Thompson, J. A. New NCCN Guidelines: Recognition and Management of Immunotherapy-Related Toxicity. J. Natl Compr. Canc. Netw. 16, 594–596 (2018).
Puzanov, I. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 5, 95 (2017).
Johnson, D. B. et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 375, 1749–1755 (2016).
Johnson, D. B. & Balko, J. M. Biomarkers for immunotherapy toxicity: are cytokines the answer? Clin. Cancer Res. 25, 1452–1454 (2019).
Johnson, D. B. et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J. Immunother. Cancer 7, 134 (2019).
Johnson, D. B. et al. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol. 21, e398–e404 (2020).
Kennedy, L. B. & Salama, A. K. S. A review of immune-mediated adverse events in melanoma. Oncol. Ther. 7, 101–120 (2019).
Weber, J. S. et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35, 785–792 (2017).
Roth, P. et al. Neurological complications of cancer immunotherapy. Cancer Treat. Rev. 97, 102189 (2021).
Morgan, R. A. et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 36, 133–151 (2013).
Acharya, U. H. et al. Management of cytokine release syndrome and neurotoxicity in chimeric antigen receptor (CAR) T cell therapy. Expert Rev. Hematol. 12, 195–205 (2019).
Gust, J. et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 (2017).
Locke, F. L. et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 20, 31–42 (2019).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Yu, L. et al. GD2-specific chimeric antigen receptor-modified T cells for the treatment of refractory and/or recurrent neuroblastoma in pediatric patients. J. Cancer Res. Clin. Oncol. https://doi.org/10.1007/s00432-021-03839-5 (2021).
Castel, D. et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 130, 815–827 (2015).
Solomon, D. A. et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 26, 569–580 (2016).
Misuraca, K. L., Cordero, F. J. & Becher, O. J. Pre-clinical models of diffuse intrinsic pontine glioma. Front. Oncol. 5, 172 (2015).
Dey, J. et al. A distinct Smoothened mutation causes severe cerebellar developmental defects and medulloblastoma in a novel transgenic mouse model. Mol. Cell. Biol. 32, 4104–4115 (2012).
Pei, Y. et al. An animal model of MYC-driven medulloblastoma. Cancer Cell 21, 155–167 (2012).
Huang, M. et al. Engineering genetic predisposition in human neuroepithelial stem cells recapitulates medulloblastoma tumorigenesis. Cell Stem Cell 25, 433–446 (2019).
Cordero, F. J. et al. Histone H3.3K27M represses p16 to accelerate gliomagenesis in a murine model of DIPG. Mol. Cancer Res. 15, 1243–1254 (2017).
Ng, J. M. et al. Generation of a mouse model of atypical teratoid/rhabdoid tumor of the central nervous system through combined deletion of Snf5 and p53. Cancer Res. 75, 4629–4639 (2015).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Piccioni, D. E. et al. Analysis of cell-free circulating tumor DNA in 419 patients with glioblastoma and other primary brain tumors. CNS Oncol. 8, CNS34 (2019).
Zhang, L., Li, Y., Meng, W., Ni, Y. & Gao, Y. Dynamic urinary proteomic analysis in a Walker 256 intracerebral tumor model. Cancer Med. 8, 3553–3565 (2019).
Elshafeey, N. et al. Multicenter study demonstrates radiomic features derived from magnetic resonance perfusion images identify pseudoprogression in glioblastoma. Nat. Commun. 10, 3170 (2019).
Schwalbe, E. C. et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a cohort study. Lancet Oncol. 18, 958–971 (2017).
Kreiter, S. et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015).
Pajtler, K. W. et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 133, 5–12 (2017).
Buczkowicz, P. et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat. Genet. 46, 451–456 (2014).
Donson, A. M. et al. Immune gene and cell enrichment is associated with a good prognosis in ependymoma. J. Immunol. 183, 7428–7440 (2009).
Doucette, T. et al. Immune heterogeneity of glioblastoma subtypes: extrapolation from the Cancer Genome Atlas. Cancer Immunol. Res. 1, 112–122 (2013).
Davis, A. A. & Patel, V. G. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 7, 278 (2019).
Rozeman, E. A. & Blank, C. U. Combining checkpoint inhibition and targeted therapy in melanoma. Nat. Med. 25, 879–882 (2019).
Deng, J. et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 8, 216–233 (2018).
Franchimont, D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann. NY Acad. Sci. 1024, 124–137 (2004).
Maxwell, R. et al. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. Oncoimmunology 7, e1500108 (2018).
Pan, E. Y., Merl, M. Y. & Lin, K. The impact of corticosteroid use during anti-PD1 treatment. J. Oncol. Pharm. Pract. 26, 814–822 (2020).
Zhu, X. et al. Severe cerebral edema following nivolumab treatment for pediatric glioblastoma: case report. J. Neurosurg. Pediatr. 19, 249–253 (2017).
Hwang, E. et al. Outcome of patients with recurrent diffuse intrinsic pontine glioma (DIPG) treated with pembrolizumab (anti-PD-1): a pediatric brain tumor consortium study (PBTC045). Neuro Oncol. 20, i100 (2018).
Okada, H. et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 16, e534–e542 (2015).
Ceschin, R. et al. Parametric response mapping of apparent diffusion coefficient as an imaging biomarker to distinguish pseudoprogression from true tumor progression in peptide-based vaccine therapy for pediatric diffuse intrinsic pontine glioma. AJNR Am. J. Neuroradiol. 36, 2170–2176 (2015).
Bloch, O. et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res. 19, 3165–3175 (2013).
Pham, C. D. et al. Differential immune microenvironments and response to immune checkpoint blockade among molecular subtypes of murine medulloblastoma. Clin. Cancer Res. 22, 582–595 (2016).
Nguyen, A. T. et al. Evidence for BRAF V600E and H3F3A K27M double mutations in paediatric glial and glioneuronal tumours. Neuropathol. Appl. Neurobiol. 41, 403–408 (2015).
Duan, S. et al. PTEN deficiency reprogrammes human neural stem cells towards a glioblastoma stem cell-like phenotype. Nat. Commun. 6, 10068 (2015).
Bian, S. et al. Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods 15, 631–639 (2018).
Ogawa, J., Pao, G. M., Shokhirev, M. N. & Verma, I. M. Glioblastoma model using human cerebral organoids. Cell Rep. 23, 1220–1229 (2018).
Koga, T. et al. Longitudinal assessment of tumor development using cancer avatars derived from genetically engineered pluripotent stem cells. Nat. Commun. 11, 550 (2020).
Monje, M. et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc. Natl Acad. Sci. USA 108, 4453–4458 (2011).
Hashizume, R. et al. Characterization of a diffuse intrinsic pontine glioma cell line: implications for future investigations and treatment. J. Neurooncol. 110, 305–313 (2012).
Harutyunyan, A. S. et al. H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis. Nat. Commun. 10, 1262 (2019).
Chen, Z. et al. Advanced pediatric diffuse pontine glioma murine models pave the way towards precision medicine. Cancers 13, 1114 (2021).
Lan, X. et al. Modeling human pediatric and adult gliomas in immunocompetent mice through costimulatory blockade. Oncoimmunology 9, 1776577 (2020).
Čančer, M. et al. Humanized stem cell models of pediatric medulloblastoma reveal an Oct4/mTOR axis that promotes malignancy. Cell Stem Cell 25, 855–870.e11 (2019).
Badodi, S. et al. Convergence of BMI1 and CHD7 on ERK signaling in medulloblastoma. Cell Rep. 21, 2772–2784 (2017).
Shiraishi, R. & Kawauchi, D. Epigenetic regulation in medulloblastoma pathogenesis revealed by genetically engineered mouse models. Cancer Sci. 112, 2948–2957 (2021).
Theruvath, J. et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat. Med. 26, 712–719 (2020).
Terada, Y. et al. Human pluripotent stem cell-derived tumor model uncovers the embryonic stem cell signature as a key driver in atypical teratoid/rhabdoid tumor. Cell Rep. 26, 2608–2621.e6 (2019).
Smith, K. S. et al. Patient-derived orthotopic xenografts of pediatric brain tumors: a St. Jude resource. Acta Neuropathol. 140, 209–225 (2020).
Huq, S. et al. Preclinical efficacy of ribavirin in SHH and group 3 medulloblastoma. J. Neurosurg. Pediatr. 5, 1–7 (2021).
Andradas, C. et al. Assessment of cannabidiol and Δ9-tetrahydrocannabiol in mouse models of medulloblastoma and ependymoma. Cancers 13, 330 (2021).
Yu, L. et al. A clinically relevant orthotopic xenograft model of ependymoma that maintains the genomic signature of the primary tumor and preserves cancer stem cells in vivo. Neuro Oncol. 12, 580–594 (2010).
Haydar, D. et al. Cell-surface antigen profiling of pediatric brain tumors: B7-H3 is consistently expressed and can be targeted via local or systemic CAR T-cell delivery. Neuro Oncol. 23, 999–1011 (2021).
Lenting, K., Verhaak, R., Ter Laan, M., Wesseling, P. & Leenders, W. Glioma: experimental models and reality. Acta Neuropathol. 133, 263–282 (2017).
Russell, W. L. et al. Specific-locus test shows ethylnitrosourea to be the most potent mutagen in the mouse. Proc. Natl Acad. Sci. USA 76, 5818–5819 (1979).
Mukherjee, J., Ghosh, A., Ghosh, A. & Chaudhuri, S. ENU administration causes genomic instability along with single nucleotide polymorphisms in p53 during gliomagenesis: T11TS administration demonstrated in vivo apoptosis of these genetically altered tumor cells. Cancer Biol. Ther. 5, 156–164 (2006).
Zook, B. C., Simmens, S. J. & Jones, R. V. Evaluation of ENU-induced gliomas in rats: nomenclature, immunochemistry, and malignancy. Toxicol. Pathol. 28, 193–201 (2000).
Koschmann, C. et al. Characterizing and targeting PDGFRA alterations in pediatric high-grade glioma. Oncotarget 7, 65696–65706 (2016).
Paugh, B. S. et al. Novel oncogenic PDGFRA mutations in pediatric high-grade gliomas. Cancer Res. 73, 6219–6229 (2013).
Puputti, M. et al. Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol. Cancer Res. 4, 927–934 (2006).
de Vries, N. A. et al. Rapid and robust transgenic high-grade glioma mouse models for therapy intervention studies. Clin. Cancer Res. 16, 3431–3441 (2010).
Holland, E. C. A mouse model for glioma: biology, pathology, and therapeutic opportunities. Toxicol. Pathol. 28, 171–177 (2000).
Bardella, C. et al. Expression of Idh1R132H in the murine subventricular zone stem cell niche recapitulates features of early gliomagenesis. Cancer Cell 30, 578–594 (2016).
Uhrbom, L., Hesselager, G., Nistér, M. & Westermark, B. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 58, 5275–5279 (1998).
Fisher, G. H. et al. Development of a flexible and specific gene delivery system for production of murine tumor models. Oncogene 18, 5253–5260 (1999).
Hede, S. M. et al. GFAP promoter driven transgenic expression of PDGFB in the mouse brain leads to glioblastoma in a Trp53 null background. Glia 57, 1143–1153 (2009).
Zhu, Y. et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell 8, 119–130 (2005).
Kwon, C. H. et al. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 68, 3286–3294 (2008).
Yu, K. et al. PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 578, 166–171 (2020).
Funato, K., Major, T., Lewis, P. W., Allis, C. D. & Tabar, V. Use of human embryonic stem cells to model pediatric gliomas with H3.3K27M histone mutation. Science 346, 1529–1533 (2014).
Pathania, M. et al. H3.3K27M cooperates with Trp53 loss and PDGFRA gain in mouse embryonic neural progenitor cells to induce invasive high-grade gliomas. Cancer Cell 32, 684–700.e9 (2017).
Larson, J. D. et al. Histone H3.3 K27M accelerates spontaneous brainstem glioma and drives restricted changes in bivalent gene expression. Cancer Cell 35, 140–155.e7 (2019).
Becher, O. et al. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res. 70, 2548–2557 (2010).
Halvorson, K. G. et al. A high-throughput in vitro drug screen in a genetically engineered mouse model of diffuse intrinsic pontine glioma identifies BMS-754807 as a promising therapeutic agent. PLoS One 10, e0118926 (2015).
Zuckermann, M. et al. Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling. Nat. Commun. 6, 7391 (2015).
Gibson, P. et al. Subtypes of medulloblastoma have distinct developmental origins. Nature 468, 1095–1099 (2010).
Robinson, G. et al. Novel mutations target distinct subgroups of medulloblastoma. Nature 488, 43–48 (2012).
Goodrich, L. V., Milenkovic, L., Higgins, K. M. & Scott, M. P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 (1997).
Lee, Y. et al. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene 26, 6442–6447 (2007).
Uziel, T. et al. The tumor suppressors Ink4c and p53 collaborate independently with patched to suppress medulloblastoma formation. Genes Dev. 19, 2656–2667 (2005).
Wetmore, C., Eberhart, D. E. & Curran, T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 61, 513–516 (2001).
Yang, Z. J. et al. Medulloblastoma can be initiated by deletion of patched in lineage-restricted progenitors or stem cells. Cancer Cell 14, 135–145 (2008).
Grammel, D. et al. Sonic hedgehog-associated medulloblastoma arising from the cochlear nuclei of the brainstem. Acta Neuropathol. 123, 601–614 (2012).
Forget, A. et al. Aberrant ERBB4-SRC signaling as a hallmark of group 4 medulloblastoma revealed by integrative phosphoproteomic profiling. Cancer Cell 34, 379–395.e7 (2018).
Hallahan, A. R. et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 64, 7794–7800 (2004).
Schüller, U. et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell 14, 123–134 (2008).
Merk, D. J. et al. Opposing effects of CREBBP mutations govern the phenotype of Rubinstein-Taybi syndrome and adult SHH medulloblastoma. Dev. Cell 44, 709–724.e706 (2018).
Shi, X., Wang, Q., Gu, J., Xuan, Z. & Wu, J. I. SMARCA4/Brg1 coordinates genetic and epigenetic networks underlying Shh-type medulloblastoma development. Oncogene 35, 5746–5758 (2016).
Hatton, B. A. et al. The Smo/Smo model: hedgehog-induced medulloblastoma with 90% incidence and leptomeningeal spread. Cancer Res. 68, 1768–1776 (2008).
Fults, D., Pedone, C., Dai, C. & Holland, E. C. MYC expression promotes the proliferation of neural progenitor cells in culture and in vivo. Neoplasia 4, 32–39 (2002).
Jenkins, N. C. et al. Somatic cell transfer of c-Myc and Bcl-2 induces large-cell anaplastic medulloblastomas in mice. J. Neurooncol. 126, 415–424 (2016).
Dhar, S. S. et al. MLL4 is required to maintain broad H3K4me3 peaks and super-enhancers at tumor suppressor genes. Mol. Cell. 70, 825–841.e826 (2018).
Lee, C. et al. Lsd1 as a therapeutic target in Gfi1-activated medulloblastoma. Nat. Commun. 10, 332 (2019).
Han, Z. Y. et al. The occurrence of intracranial rhabdoid tumours in mice depends on temporal control of Smarcb1 inactivation. Nat. Commun. 7, 10421 (2016).
Ozawa, T. et al. A de novo mouse model of C11orf95-RELA fusion-driven ependymoma identifies driver functions in addition to NF-κB. Cell Rep. 23, 3787–3797 (2018).
Pajtler, K. W. et al. YAP1 subgroup supratentorial ependymoma requires TEAD and nuclear factor I-mediated transcriptional programmes for tumorigenesis. Nat. Commun. 10, 3914 (2019).
Eder, N. et al. YAP1/TAZ drives ependymoma-like tumour formation in mice. Nat. Commun. 11, 2380 (2020).
Acknowledgements
This publication was supported in part by the ReMission Alliance against Brain Tumors.
Author information
Authors and Affiliations
Contributions
Conceived of and collected data: E.I.H., E.J.S., C.T.F., G.G., R.W.-R., L.B.H.-M., M.W.K., J.S., R.M.P., J.W.F., M.P., K.M.C., P.G.H., J.E.B., L.S.G., H.O., D.A.M. and I.F.P. Drafted, reviewed and edited the manuscript: E.I.H., E.J.S., C.T.F., G.G., R.W.-R., L.B.H.-M., M.W.K., J.S., R.M.P., J.W.F., M.P., K.M.C., P.G.H., J.E.B., L.S.G., H.O., D.A.M. and I.F.P.
Corresponding author
Ethics declarations
Competing interests
All authors declare no conflict of interest, with the following exceptions: M.W.K. is an employee and stock holder of Day One Biopharmaceuticals and M.P. holds patents on histone anti-cancer vaccines (US20180155403A1) and on tryptophan metabolism in cancer immunotherapy (EP2753315B1 and EP3464248A1).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hwang, E.I., Sayour, E.J., Flores, C.T. et al. The current landscape of immunotherapy for pediatric brain tumors. Nat Cancer 3, 11–24 (2022). https://doi.org/10.1038/s43018-021-00319-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-021-00319-0
This article is cited by
-
Glioblastoma stem cell metabolism and immunity
Cancer and Metastasis Reviews (2024)
-
Intra-tumoral T cells in pediatric brain tumors display clonal expansion and effector properties
Nature Cancer (2024)
-
The expanding palette of immunotherapy research
Nature Cancer (2022)