Abstract
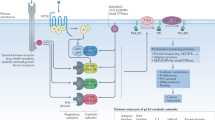
Phosphoinositide 3-kinase (PI3K) signaling regulates cellular proliferation, survival and metabolism, and its aberrant activation is one of the most frequent oncogenic events across human cancers. In the last few decades, research has focused on the development of PI3K inhibitors, from preclinical tool compounds to the highly specific medicines approved to treat patients with cancer. Herein we discuss current paradigms for PI3K inhibitors in cancer therapy, focusing on clinical data and mechanisms of action. We also discuss current limitations in the use of PI3K inhibitors, including toxicities and mechanisms of resistance, with specific emphasis on approaches aimed at improving efficacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bilanges, B., Posor, Y. & Vanhaesebroeck, B. PI3K isoforms in cell signalling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 20, 515–534 (2019).
Thorpe, L. M., Yuzugullu, H. & Zhao, J. J. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 15, 7–24 (2015).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017).
Burke, J. E. Structural basis for regulation of phosphoinositide kinases and their involvement in human disease. Mol. Cell 71, 653–673 (2018).
Vadas, O., Burke, J. E., Zhang, X., Berndt, A. & Williams, R. L. Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci. Signal. 4, re2 (2011).
Rodriguez-Viciana, P. et al. Phosphatidylinositol-3-OH kinase direct target of Ras. Nature 370, 527–532 (1994).
Gupta, S. et al. Binding of ras to phosphoinositide 3-kinase p110α is required for ras-driven tumorigenesis in mice. Cell 129, 957–968 (2007).
Park, W. S. et al. Comprehensive identification of PIP3-regulated PH domains from C. elegans to H. sapiens by model prediction and live imaging. Mol. Cell 30, 381–392 (2008).
Hoxhaj, G. & Manning, B. D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 20, 74–88 (2020).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017).
Eijkelenboom, A. & Burgering, B. M. T. FOXOs: signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 14, 83–97 (2013).
Worby, C. A. & Dixon, J. E. PTEN. Annu. Rev. Biochem. 83, 641–669 (2014).
Samuels, Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 (2004).
Miled, N. et al. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science 317, 239–242 (2007).
Burke, J. E., Perisic, O., Masson, G. R., Vadas, O. & Williams, R. L. Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110α (PIK3CA). Proc. Natl Acad. Sci. USA 109, 15259–15264 (2012).
Bailey, M. H. et al. Comprehensive characterization of cancer driver genes and mutations. Cell 173, 371–385 (2018).
Madsen, R. R., Vanhaesebroeck, B. & Semple, R. K. Cancer-associated PIK3CA mutations in overgrowth disorders. Trends Mol. Med. 24, 856–870 (2018).
Castel, P., Rauen, K. A. & McCormick, F. The duality of human oncoproteins: drivers of cancer and congenital disorders. Nat. Rev. Cancer 20, 383–397 (2020).
Lui, V. W. Y. et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 3, 761–769 (2013).
Jia, S. et al. Essential roles of PI(3)K–p110β in cell growth, metabolism and tumorigenesis. Nature 454, 776–779 (2008).
Cheung, L. W. T. et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 1, 170–185 (2011).
Elkabets, M. et al. mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci. Transl. Med. 5, 196ra99 (2013).
Will, M. et al. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS–ERK signaling. Cancer Discov. 4, 334–347 (2014).
Fritsch, C. et al. Characterization of the novel and specific PI3Kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol. Cancer Ther. 13, 1117–1129 (2014).
She, Q.-B. et al. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 8, 287–297 (2005).
Salih, D. A. M. & Brunet, A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 20, 126–136 (2008).
Yang, W. et al. Autophagy promotes escape from phosphatidylinositol 3-kinase inhibition in estrogen receptor-positive breast cancer. FASEB J. 32, 1222–1235 (2018).
Takuwa, N., Fukui, Y. & Takuwa, Y. Cyclin D1 expression mediated by phosphatidylinositol 3-kinase through mTOR–p70S6K-independent signaling in growth factor-stimulated NIH 3T3 fibroblasts. Mol. Cell. Biol. 19, 1346–1358 (1999).
Faber, A. C. et al. mTOR inhibition specifically sensitizes colorectal cancers with KRAS or BRAF mutations to BCL-2/BCL-XL inhibition by suppressing MCL-1. Cancer Discov. 4, 42–52 (2014).
Li, H., Liu, L., Chang, H., Zou, Z. & Xing, D. Downregulation of MCL-1 and upregulation of PUMA using mTOR inhibitors enhance antitumor efficacy of BH3 mimetics in triple-negative breast cancer. Cell Death Dis. 9, 137 (2018).
Soler, A., Angulo-Urarte, A. & Graupera, M. PI3K at the crossroads of tumor angiogenesis signaling pathways. Mol. Cell. Oncol. 2, e975624 (2015).
Soler, A. et al. Inhibition of the p110α isoform of PI 3-kinase stimulates nonfunctional tumor angiogenesis. J. Exp. Med. 210, 1937–1945 (2013).
Kaneda, M. M. et al. PI3Kγ is a molecular switch that controls immune suppression. Nature 539, 437–442 (2016).
Davis, R. J. et al. Anti-PD-L1 efficacy can be enhanced by inhibition of myeloid-derived suppressor cells with a selective inhibitor of PI3Kδ/γ. Cancer Res. 77, 2607–2619 (2017).
Okkenhaug, K. et al. Impaired B and T cell antigen receptor signaling in p110δ PI 3-kinase mutant mice. Science 297, 1031–1034 (2002).
Angulo, I. et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science 342, 866–871 (2013).
Ali, K. et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature 510, 407–411 (2014).
Okkenhaug, K., Graupera, M. & Vanhaesebroeck, B. Targeting PI3K in cancer: impact on tumor cells, their protective stroma, angiogenesis, and immunotherapy. Cancer Discov. 6, 1090–1105 (2016).
Hopkins, B. D. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018).
Ortega-Molina, A. et al. Pharmacological inhibition of PI3K reduces adiposity and metabolic syndrome in obese mice and rhesus monkeys. Cell Metab. 21, 558–570 (2015).
Wei, Z. et al. Death-associated protein kinase 1 (DAPK1) controls CD8+ T cell activation, trafficking, and antitumor activity. FASEB J. 35, e21138 (2021).
Ding, J., Vlahos, C. J., Liu, R., Brown, R. F. & Badwey, J. A. Antagonists of phosphatidylinositol 3-kinase block activation of several novel protein kinases in neutrophils. J. Biol. Chem. 270, 11684–11691 (1995).
Anel, A. et al. Two signaling pathways can lead to Fas ligand expression in CD8+ cytotoxic T lymphocyte clones. Eur. J. Immunol. 25, 3381–3387 (1995).
Fruman, D. A. et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science 283, 393–397 (1999).
Sanchez-Margálet, V., Goldfine, I. D., Vlahos, C. J. & Sung, C. K. Role of phosphatidylinositol-3-kinase in insulin receptor signaling: studies with inhibitor, LY294002. Biochem. Biophys. Res. Commun. 204, 446–452 (1994).
Hayakawa, J. et al. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 60, 5988–5994 (2000).
Misra, U. K. & Pizzo, S. V. Binding of receptor-recognized forms of α2-macroglobulin to the α2-macroglobulin signaling receptor activates phosphatidylinositol 3-kinase. J. Biol. Chem. 273, 13399–13402 (1998).
Graness, A., Adomeit, A., Heinze, R., Wetzker, R. & Liebmann, C. A novel mitogenic signaling pathway of bradykinin in the human colon carcinoma cell line SW-480 involves sequential activation of a Gq/11 protein, phosphatidylinositol 3-kinase β, and protein kinase Cε. J. Biol. Chem. 273, 32016–32022 (1998).
Ihle, N. T. et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol. Cancer Ther. 3, 763–772 (2004).
Folkes, A. J. et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 51, 5522–5532 (2008).
Junttila, T. T. et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 15, 429–440 (2009).
Maira, S.-M. et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol. Cancer Ther. 11, 317–328 (2012).
Sanchez, C. G. et al. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res. 13, R21 (2011).
Miller, T. W. et al. ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 1, 338–351 (2011).
Bendell, J. C. et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 30, 282–290 (2012).
Mayer, I. A. et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 32, 1202–1209 (2014).
Baselga, J. et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 904–916 (2017).
Geuna, E. et al. Buparlisib, an oral pan-PI3K inhibitor for the treatment of breast cancer. Expert Opin. Investig. Drugs 24, 421–431 (2015).
Serra, V. et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 68, 8022–8030 (2008).
Rexer, B. N., Chanthaphaychith, S., Dahlman, K. & Arteaga, C. L. Direct inhibition of PI3K in combination with dual HER2 inhibitors is required for optimal antitumor activity in HER2+ breast cancer cells. Breast Cancer Res. 16, R9 (2014).
Saura, C. et al. Phase Ib study of buparlisib plus trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on trastuzumab-based therapy. Clin. Cancer Res. 20, 1935–1945 (2014).
Loibl, S. et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: a randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE). Eur. J. Cancer 85, 133–145 (2017).
Pistilli, B. et al. Phase II study of buparlisib (BKM120) and trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer resistant to trastuzumab-based therapy. Breast Cancer Res. Treat. 168, 357–364 (2018).
Ibrahim, Y. H. et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2, 1036–1047 (2012).
Juvekar, A. et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2, 1048–1063 (2012).
Liu, N. et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol. Cancer Ther. 12, 2319–2330 (2013).
Dreyling, M. et al. Phosphatidylinositol 3-Kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J. Clin. Oncol. 35, 3898–3905 (2017).
Matasar, M. J. et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(21)00145-5 (2021).
Furet, P. et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase α inhibitor selected for clinical evaluation. Bioorg. Med. Chem. Lett. 23, 3741–3748 (2013).
Juric, D. et al. Phosphatidylinositol 3-kinase α-selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: results from the first-in-human study. J. Clin. Oncol. 36, 1291–1299 (2018).
Juric, D. et al. Alpelisib plus fulvestrant in PIK3CA-altered and PIK3CA-wild-type estrogen receptor-positive advanced breast cancer: a phase 1b clinical trial. JAMA Oncol. 5, e184475 (2019).
André, F. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380, 1929–1940 (2019).
Ndubaku, C. O. et al. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1 H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a β-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J. Med. Chem. 56, 4597–4610 (2013).
Saura, C. et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2-negative, early-stage breast cancer (LORELEI): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 20, 1226–1238 (2019).
Juric, D. et al. Phase I dose-escalation study of taselisib, an oral PI3K inhibitor, in patients with advanced solid tumors. Cancer Discov. 7, 704–715 (2017).
Jhaveri, K. et al. Phase I basket study of taselisib, an isoform-selective PI3K inhibitor, in patients with PIK3CA-mutant cancers. Clin. Cancer Res. 27, 447–459 (2021).
Zumsteg, Z. S. et al. Taselisib (GDC-0032), a potent β-sparing small molecule inhibitor of PI3K, radiosensitizes head and neck squamous carcinomas containing activating PIK3CA alterations. Clin. Cancer Res. 22, 2009–2019 (2016).
Hong, R. et al. Abstract PD4-14: GDC-0077 is a selective PI3Kα inhibitor that demonstrates robust efficacy in PIK3CA mutant breast cancer models as a single agent and in combination with standard of care therapies. Cancer Res. https://doi.org/10.1158/1538-7445.SABCS17-PD4-14 (2018).
Furman, R. R. et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 370, 997–1007 (2014).
Flinn, I. W. et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 132, 2446–2455 (2018).
Fowler, N. H. et al. Umbralisib, a dual PI3Kδ/CK1ε inhibitor in patients with relapsed or refractory indolent lymphoma. J. Clin. Oncol. https://doi.org/10.1200/JCO.20.03433 (2021).
Castel, P. et al. Somatic PIK3CA mutations as a driver of sporadic venous malformations. Sci. Transl. Med. 8, 332ra42 (2016).
Venot, Q. et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 558, 540–546 (2018).
Hanker, A. B., Kaklamani, V. & Arteaga, C. L. Challenges for the clinical development of PI3K inhibitors: strategies to improve their impact in solid tumors. Cancer Discov. 9, 482–491 (2019).
Razavi, P. et al. Alterations in PTEN and ESR1 promote clinical resistance to alpelisib plus aromatase inhibitors. Nat. Cancer 1, 382–393 (2020).
Toska, E. & Baselga, J. Pharmacology in the era of targeted therapies: the case of PI3K inhibitors. Clin. Cancer Res. 22, 2099–2101 (2016).
Yang, W. et al. Strategically timing inhibition of phosphatidylinositol 3-kinase to maximize therapeutic index in estrogen receptor α-positive, PIK3CA-mutant breast cancer. Clin. Cancer Res. 22, 2250–2260 (2016).
Juric, D. et al. A first-in-human, phase I, dose-escalation study of TAK-117, a selective PI3Kα isoform inhibitor, in patients with advanced solid malignancies. Clin. Cancer Res. 23, 5015–5023 (2017).
Lai, A. C. & Crews, C. M. Induced protein degradation: an emerging drug discovery paradigm. Nat. Rev. Drug Discov. 16, 101–114 (2017).
Fischer, E. S., Park, E., Eck, M. J. & Thomä, N. H. SPLINTs: small-molecule protein ligand interface stabilizers. Curr. Opin. Struct. Biol. 37, 115–122 (2016).
Siempelkamp, B. D., Rathinaswamy, M. K., Jenkins, M. L. & Burke, J. E. Molecular mechanism of activation of class IA phosphoinositide 3-kinases (PI3Ks) by membrane-localized HRas. J. Biol. Chem. 292, 12256–12266 (2017).
Vora, S. R. et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 26, 136–149 (2014).
Pascual, J. et al. Triplet therapy with palbociclib, taselisib, and fulvestrant in PIK3CA-mutant breast cancer and doublet palbociclib and taselisib in pathway-mutant solid cancers. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-20-0553 (2020).
Soulières, D. et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 18, 323–335 (2017).
Engelman, J. A. et al. Effective use of PI3K and MEK inhibitors to treat mutant K-Ras G12D and PIK3CA H1047R murine lung cancers. Nat. Med. 14, 1351–1356 (2008).
Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. K-RasG12C inhibitors allosterically control GTP affinity and effector interactions. Nature 503, 548–551 (2013).
Misale, S. et al. KRAS G12C NSCLC models are sensitive to direct targeting of KRAS in combination with PI3K inhibition. Clin. Cancer Res. 25, 796–807 (2019).
Vasan, N., Baselga, J. & Hyman, D. M. A view on drug resistance in cancer. Nature 575, 299–309 (2019).
O’Reilly, K. E. et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66, 1500–1508 (2006).
Chandarlapaty, S. et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19, 58–71 (2011).
Serra, V. et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene 30, 2547–2557 (2011).
Chakrabarty, A., Sánchez, V., Kuba, M. G., Rinehart, C. & Arteaga, C. L. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc. Natl Acad. Sci. USA 109, 2718–2723 (2012).
Tao, J. J. et al. Antagonism of EGFR and HER3 enhances the response to inhibitors of the PI3K–Akt pathway in triple-negative breast cancer. Sci. Signal. 7, ra29 (2014).
Wong, M. H. et al. Cotargeting of epidermal growth factor receptor and PI3K overcomes PI3K–Akt oncogenic dependence in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 20, 4047–4058 (2014).
Juric, D. et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 518, 240–244 (2015).
Costa, C. et al. Measurement of PIP3 levels reveals an unexpected role for p110β in early adaptive responses to p110α-specific inhibitors in luminal breast cancer. Cancer Cell 27, 97–108 (2015).
Schwartz, S. et al. Feedback suppression of PI3Kα signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kβ. Cancer Cell 27, 109–122 (2015).
Elkabets, M. et al. AXL mediates resistance to PI3Kα inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell 27, 533–546 (2015).
Castel, P. et al. PDK1–SGK1 signaling sustains AKT-independent mTORC1 activation and confers resistance to PI3Kα inhibition. Cancer Cell 30, 229–242 (2016).
Bago, R. et al. The hVps34–SGK3 pathway alleviates sustained PI3K/Akt inhibition by stimulating mTORC1 and tumour growth. EMBO J. 35, 1902–1922 (2016).
Cai, Y. et al. Genomic alterations in PIK3CA-mutated breast cancer result in mTORC1 activation and limit sensitivity to PI3Kα inhibitors. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-20-3232 (2021).
Le, X. et al. Systematic functional characterization of resistance to PI3K inhibition in breast cancer. Cancer Discov. 6, 1134–1147 (2016).
Ros, S. et al. Metabolic imaging detects resistance to PI3Kα inhibition mediated by persistent FOXM1 expression in ER+ breast cancer. Cancer Cell 38, 516–533 (2020).
Bosch, A. et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci. Transl. Med. 7, 283ra51 (2015).
Ciriello, G. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163, 506–519 (2015).
Toska, E. et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science 355, 1324–1330 (2017).
Toska, E. et al. PI3K inhibition activates SGK1 via a feedback loop to promote chromatin-based regulation of ER-dependent gene expression. Cell Rep. 27, 294–306 (2019).
Carver, B. S. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 (2011).
de Bono, J. S. et al. Randomized phase II study evaluating Akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin. Cancer Res. 25, 928–936 (2019).
Di Leo, A. et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 19, 87–100 (2018).
Gopal, A. K. et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N. Engl. J. Med. 370, 1008–1018 (2014).
Salles, G. et al. Efficacy and safety of idelalisib in patients with relapsed, rituximab- and alkylating agent-refractory follicular lymphoma: a subgroup analysis of a phase 2 study. Haematologica 102, e156–e159 (2017).
Juric, D. et al. Abstract OT1-08-04: A first-in-human phase Ia dose escalation study of GDC-0077, a p110a-selective and mutant-degrading PI3K inhibitor, in patients with PIK3CA-mutant solid tumors. Cancer Res. https://doi.org/10.1158/1538-7445.SABCS19-OT1-08-04 (2020).
Kalinsky, K. et al. Abstract CT109: A phase I/Ib study evaluating GDC-0077 plus fulvestrant in patients with PIK3CA-mutant, hormone receptor-positive/HER2-negative breast cancer. Cancer Res. https://doi.org/10.1158/1538-7445.AM2020-CT109 (2020).
Acknowledgements
We dedicate this Review to the memory of José Baselga, who was one of the pioneers in the clinical development of PI3K inhibitors. The work of P.C. is supported by an NCI K99/R00 Pathway to Independence Award (K99CA245122). E.T. is supported by grants from the JKTG Foundation, the Breast Cancer Research Alliance, the Institute of Cancer Informatics and an NCI K22 Transition to Independence Award. M.S. is supported by NIH grant P30CA008748.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
P.C. is a founder and advisory board member of Venthera. E.T. has received honoraria for invited lectures at AstraZeneca and Oric Pharmaceuticals. M.S. is an AstraZeneca employee, holds AstraZeneca equity and is a cofounder of medendi.org. In the past 2 years, M.S. received research funding from Daiichi-Sankio, AstraZeneca, Menarini Ricerche, Puma Biotechnologies and Targimmune. J.A.E. was a Novartis employee and holds Novartis equity. J.A.E. is a cofounder of Treeline Biosciences.
Additional information
Peer review information Nature Cancer thanks Filip Janku, Cynthia Ma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Castel, P., Toska, E., Engelman, J.A. et al. The present and future of PI3K inhibitors for cancer therapy. Nat Cancer 2, 587–597 (2021). https://doi.org/10.1038/s43018-021-00218-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-021-00218-4
This article is cited by
-
A novel pan-PI3K inhibitor KTC1101 synergizes with anti-PD-1 therapy by targeting tumor suppression and immune activation
Molecular Cancer (2024)
-
Tumor location matters, next generation sequencing mutation profiling of left-sided, rectal, and right-sided colorectal tumors in 552 patients
Scientific Reports (2024)
-
Microarray-based detection and expression analysis of drug resistance in an animal model of peritoneal metastasis from colon cancer
Clinical & Experimental Metastasis (2024)
-
Revolutionizing cancer care strategies: immunotherapy, gene therapy, and molecular targeted therapy
Molecular Biology Reports (2024)
-
NF-κB signaling in neoplastic transition from epithelial to mesenchymal phenotype
Cell Communication and Signaling (2023)