The risks posed to patients with cancer by the current COVID-19 pandemic demand rapid structural changes in healthcare delivery, with many positive changes likely to continue long term. An immediate critical reassessment of trial methodology based on clinical and scientific priorities is essential to ensure the resilience of clinical cancer research and optimize patient-centered care.
The COVID-19 pandemic is challenging clinical cancer research and care profoundly. This is especially true for interventional oncology clinical trials in which patient populations may be highly vulnerable to COVID-19 morbidity and mortality and in which treatment strategies may increase risk yet further. As a result, accepted optimal standards of care have shifted rapidly in response to the pandemic, with physicians, healthcare systems and patients attempting to mitigate uncertain risks to develop pragmatic anti-cancer treatment strategies1.
Although different countries are at different pandemic ‘stages’, with distinct demographics, societal structures and methods for minimizing the population risk from COVID-19, for patients with cancer, every hospital visit or treatment can present an additional dynamic, yet incalculable, safety risk. This presents a potential paradox for clinical research, for which more-frequent hospital visits, with exhaustive and frequent trial assessments, have hitherto been deemed to optimize patient safety.
Technological advances and the ongoing shift in standard-of-care treatment and safety strategies, toward generally less-intensive yet patient-centered care, necessitate a re-examination of what patient safety now means in a world of virtual visits2, electronic patient-reported outcomes (ePROs)3, frequent cross-sectional imaging, high-quality blood tests, novel medical devices, including wearable technologies4, and highly targeted radiotherapy and drugs with known class effects and greater safety and tolerability in general than that of historical agents.
Given that the pandemic and its consequences are unlikely to dissipate soon, now is the right time to fundamentally rethink study designs, including the reasoning behind every study visit and intervention, in order to rationalize and optimize clinical cancer research. Here we present potential solutions for sponsors, investigators and regulatory agencies both for ongoing trials during the pandemic and to aid the design and implementation of future trials.
Adaptations of clinical care and research in response to COVID-19
Institutions offering clinical trials typically embed research into normal practice settings, reflective of the normalization of research participation, with trial treatments becoming bona fide treatment lines. Oncologists now routinely use a wide variety of drug classes, managing dosing and myriad toxicities to balance patient safety and anti-cancer efficacy for patients in trials and those not in trials. Although patients in trials might be assumed to be at greater risk than those not in trials, given the uncertainty of novel treatments or combinations, the latter are also exposed to certain risks from standard-of-care treatments, which are often substantial yet do not often mandate trial-like patient-monitoring intensity. Therefore, considering successful adaptations to standard-of-care management that optimize patient safety and anti-cancer efficacy during the COVID-19 pandemic may also be informative about positive adaptations to clinical trial protocols.
National and international clinical authorities, including NHS England, ESMO and ASCO, have released recommendations for how anti-cancer treatment should be prioritized or adapted during the COVID-19 pandemic. The consensus is to focus on treatments that provide greatest clinical benefit, to reduce potential risk to patients by adapting treatment regimens and to lessen COVID-19 exposure by minimizing hospital visits. Physicians and patients have quickly adapted to new ways of interacting (e.g., through telephone or video consultations2) and new ways of perceiving and balancing risks (e.g., reducing the number of blood tests or imaging evaluations, or altering treatment regimens, including avoiding immunosuppressive drugs and those with a propensity to cause pneumonitis). Some adjustments are evidence based, while others are simply rational or pragmatic and therefore have uncertain short- and long-term impact. Given these uncertainties, such adjustments are therefore best made through shared decision-making with patients, with due regard to the local situation and outlook.
Many physicians and patients hope that certain adaptations made in response to COVID-19 will continue beyond the pandemic and will stimulate further innovation in healthcare and research delivery. These changes present an opportunity for new trials to more closely mirror new real-world treatment standards and methods while still ensuring trial integrity and scientific validity and, in parallel, addressing concerns that clinical trial populations do not closely resemble real-world populations treated for the same condition. The extent to which changes should be implemented will vary by trial type and phase. For first-in-human, dose-finding and new drug-class trials, some adaptations may be harder to implement, given the balance of competing risks, but should still be considered. Conversely, for randomized phase 3 trials with a standard-of-care comparator, the study design should maximize translatability to routine practice. Furthermore, patients may understandably not be attracted to randomized trials with a ‘standard-of-care’ arm with a greater intensity of study assessments and hospital visits than normal practice. All efforts must be made to ensure trials are as patient centered as possible, and this will help increase research efficiency.
National regulatory authorities have responded rapidly by facilitating protocol flexibility and adaptations to ongoing trials during the COVID-19 pandemic, ensuring that these trials can continue in a safe and patient-centered manner5. Many study centers have stopped enrollment into ongoing studies and have stopped opening new studies, with considerable uncertainty about how long restrictions will continue. Some major challenges for clinical cancer research will persist long term. Therefore, ‘future-proofing’ of trials must be considered on the basis of the assumption that the COVID-19 challenge might not fully dissipate and that further zoonotic disease may emerge. Robust adaptations should render the field more resilient to future pandemics. However, it remains important to distinguish between trial-protocol adaptations that should occur regardless of COVID-19 and contingency measures that should come into effect if perceived risks for certain protocolized trial visits or assessments become too high. Where these do not fully coincide, a robust contingency plan should be included prospectively in new and ongoing trials.
Considerations for trial protocol adaptations
Here we present specific recommendations for adapting clinical trial protocols to mitigate COVID-19-related risks while modernizing and ‘future-proofing’ research, with an emphasis on patient safety and preservation of scientific integrity. We encourage regulatory authorities to be flexible in permitting these approaches where justified. These are summarized in Fig. 1.
Recommendations for trial sponsors, investigators and regulatory authorities for modernizing and ‘future-proofing’ clinical cancer trials. Considerations that apply to clinical trial design and implementation are shown as a timeline, with recommendations for changes and improvements shown in boxes for each step. ctDNA, circulating tumor DNA; HCW, healthcare worker; IMP, investigational medicinal product.
Informed-consent procedures
Informed consent (IC) requires a full, open discussion that includes the trial’s aims, procedures and associated risks, with the opportunity for patients to ask questions of the investigator. This is traditionally documented by the signing and dating of a paper IC form by the patient and investigator. Some centers have already moved to documenting IC electronically6, a process that has been endorsed by certain regulators. Using remote-visit technology and electronic signatures to avoid unnecessary hospital visits would improve IC efficiency without sacrificing quality. The process should be flexible, given the range of technological options potentially available, patients’ heterogeneous digital literacy7 and necessary contingencies. The focus should be on outcomes rather than process, ensuring verification of a patient’s identity and that the consent is given freely and is valid, as well as determination and recording of precisely what the patient has given consent for.
Screening assessments
Screening assessments determine trial eligibility and serve as a baseline for the patient’s status, to which ‘on-trial’ events can be compared. There are two main purposes for study eligibility criteria: (i) to determine if the patient has the particular characteristics to contribute to optimal, formal testing of the study hypothesis; and (ii) to determine if it is deemed safe for the patient to proceed with the study. Although patient characteristics should ideally mimic those of the real-world population to which results might be applied, they often do not, and eligibility criteria are often exhaustive and restrictive. For example, mandating a minimum estimated creatinine clearance for a renally excreted trial drug is rational, but such a restriction for drugs for which renal clearance is known not to occur is less so, unless justifiable for other reasons. Labor-intensive assessments are often included with absent or weak justification (e.g., echocardiograms or MUGA scans in patients asymptomatic of cardiac disease in trials in which no previous cardiac safety signal has been observed or would reasonably be expected). ASCO has produced guidelines that argue strongly for more-permissive eligibility criteria8. All attempts should be made to schedule required screening assessments during the same hospital visit (and in direct sequence), to maximize patient convenience and minimize infection risks. Such eligibility and screening adjustments are likely to increase patient enrollment and thereby increase research efficiency while more closely reflecting real-world populations and maximizing translatability.
Ongoing safety assessments
Ongoing safety assessments are important for the detection of clinically relevant (including otherwise silent) toxicities or disease complications. Methods and frequencies of safety assessments should be rationally determined in trial protocols, be scientifically and ethically justified and consider both the inconvenience and risks associated with hospital visits. Physical examination or other close contact with healthcare workers can pose particular risks to patients, given that there is asymptomatic carriage of COVID-199. Some assessments are of questionable value (especially those with high inter-operator variability) or are even redundant if cross-sectional imaging is performed in parallel. Any perceived ‘risk increases’ here (by losing potential safety information) could be balanced by ‘risk decreases’ elsewhere (e.g., by monitoring a drug-induced rash daily via video technology, or increasing the frequency of symptom assessment via ePROs). Other tests could be replaced by patients performing assessments at home after appropriate training, such as obtaining vital signs. Wearable technologies that can continuously detect routine safety parameters, including electrical cardiac monitoring (with QTc measurement10), can potentially highlight clinically important issues not readily ascertained during infrequent hospital-based assessments; this would provide high-yield safety information.
Blood sampling for monitoring of hematological and organ function is unlikely to be replaced any time soon. However, venipuncture at home, or closer to home with local providers, or even blood sampling by patients themselves via skin pricks, could allow patients to avoid study-center visits while still allowing accredited analysis. Tests for which, in the absence of relevant symptoms, abnormal results do not change management should be minimized unless scientifically justified. Safety-assessment choices should take into account pre-clinical and clinical safety data for the trial treatment(s), safety data for the relevant class(es) of agents, alternative methods of obtaining satisfactory safety data, and the rapidity by which patients can report new symptoms on trial. ePROs and validated questionnaires, with appropriate face-to-face follow-up for any relevant items of concern, are pragmatic and patient centered and may improve adverse-event reporting and even survival11. Protocolized safety assessments that must occur in study centers should be scheduled so as to minimize the number and length of visits and minimize other risks (e.g., by obtaining necessary intravenous access and blood samples with a single procedure, immediately before efficacy assessment). For many trials, the extent to which chosen assessment frequencies are evidence based or merely reproduce schedules from similar trials is unclear; each assessment must be critically examined. For trials for which specific concerns arise, additional or altered assessments and schedules can be easily instituted as required.
Efficacy assessments
Typically, oncology clinical trials have mandated more (usually imaging-based) efficacy assessments than occur during routine practice. Often these must be performed within narrow, inflexible time windows. Whereas inflexibility can assist with trial endpoint assessment, precise timings can sometimes be difficult to accommodate regardless of the ongoing pandemic. Rigid assessment timings often lead to stepwise Kaplan-Meier curves, which makes their interpretation sometimes challenging. Two main competing risks must be balanced: (i) risks from undocumented cancer progression and continuing ineffective treatment(s) associated with toxicity; and (ii) risks from the frequency of hospital visits. The optimal frequency and flexibility of efficacy assessments depends on the trial design and the agent(s) used. While information at very early time points can be scientifically useful, it may be less so clinically if the results are unlikely to change management (e.g., if early progression requires later confirmation and/or if treatment is continued regardless). Efficacy surrogates, including circulating tumor DNA (which may even be captured by the patient at home via dried blood spots12) and circulating tumor markers, may reduce the need for cross-sectional imaging. Traditionally, deterioration in clinical status triggers cross-sectional imaging. ePROs could function as imaging-assessment triggers, perhaps with greater clinical relevance than imaging at standard time points. Efficacy assessments could be performed with other providers, closer to the patient’s home or in an area of lower risk, and could be sent to the study center for formal reporting. Ensuring agreement from, and reimbursement of, such providers for efficacy and safety assessments may be challenging to set up and administer; this may be aided by permitting oncologists in these centers to be trained as sub-investigators with delegated duties under the supervision of the main study team. If such research networks are established and permitted by study sponsors and regulators, this will help democratize research participation and enhance research efficiency.
General trial procedures and considerations
A typical day for a patient in a clinical trial at the start of a new treatment cycle involves visiting the study center for vital-sign measurements, blood tests, electrocardiogram(s), adverse-event determination, physical examination, performance-status determination, medication review, questionnaire or diary collection, return of unused medication, and any other trial-specific special tests or investigations. The patient must usually wait until the study physician can prescribe the next cycle, if appropriate after all the outcomes of the above are available, and wait further for drug dispensing (and even administration). This can take many hours and presents unnecessary infection risks. While these visits are intended to optimize patient safety and data collection, for many trials these can be optimized through the use of alternative methods, such as virtual visits, blood tests at home before trial prescription, wearable technologies, and ePROs, questionnaires and/or diary cards. For oral medication, a physical visit to the study center may not even be required. Truly necessary ‘in-person’ safety assessments and study interventions should be performed as part of a short visit. This could help free up trial-unit capacity so more patients can be managed. Couriers could transfer any required documents and oral or other self-administered medication. Even parenteral trial medication could potentially be administered at home13. Minimizing hospital contact may increase the recruitment of patients who live further from study centers, which would democratize trial participation. In early-phase clinical trials, in which sponsors, investigators and protocols are understandably more risk averse, some of these measures could still be adopted to balance competing risks. In later-phase trials, collection of pharmacokinetic and/or pharmacodynamic data should stop once sufficient data have been collected, to spare patients enrolled later in the trial often long study visits, without jeopardizing scientific outcomes.
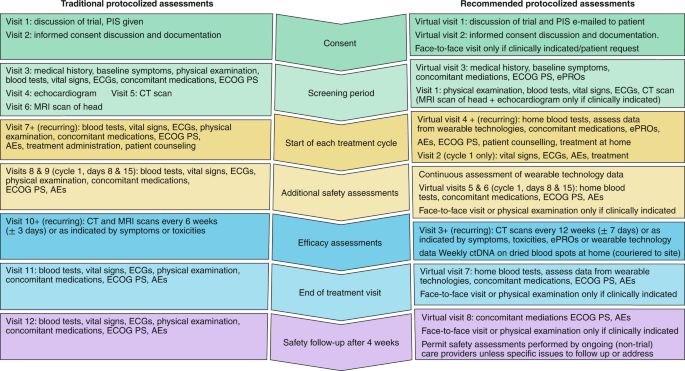
An illustrative example with a side-by-side comparison of traditional and adapted trial protocol assessments is presented in Fig. 2.
Side-by-side comparison of a traditional interventional cancer clinical trial assessment schedule with implications for patients, and the suggested protocol adaptations that might result in clinical cancer research that is more patient-centered, resilient and efficient. PIS, participant information sheet; ECG, electrocardiogram; ECOG PS, Eastern Cooperative Oncology Group Performance Status; CT, computed tomography; AEs, adverse events; MRI, magnetic resonance imaging.
Specific considerations from the COVID-19 crisis
COVID-19 presents specific challenges to clinical research for study centers, investigators and patients. The precise risks posed to specific cohorts of patients with cancer remain unknown and will become clearer in the next few months with prospective registry studies, including the UK Coronavirus Cancer Monitoring Project, although early indications suggest a particularly high risk to some patients. Potential risks associated with COVID-19 must be discussed with new patients at the time IC is provided, and with ongoing patients at the next available opportunity, contextualized with the patient’s own background risk.
Many study centers are halting trial enrollment and delaying the opening of new trials14. A shortage of study personnel on site, due to staff redeployment, shielding or illness, affects the ability to deliver clinical trials safely and to minimize risks for patients and staff. The geographical variance of the pandemic and distinct methods used by countries to contain their own (often regional) epidemics will make COVID-19 risk assessment for clinical research a local issue for study sites, at least for the time being. It seems prudent not to open new trials while there is a rising or high prevalence of COVID-19. The optimal strategies and timings for restarting normal trial activity remain unclear. However, progress in cancer research must continue. In areas in which COVID-19 is epidemic or endemic, study centers should ideally have a low-risk area for patients in trials, where all patients and staff who enter are screened for common COVID-19 symptoms and fever. All patients and staff should wear appropriate personal protective equipment. The value of screening potential or ongoing study participants for COVID-19 through the use of RT-PCR, antigen and/or serology tests is currently unclear, but such screening seems prudent where available, as does regular testing of staff. It is unclear how patients with COVID-19-like imaging changes, symptoms or confirmed infection on trial (or during screening) should be managed — protocols should include trial-specific guidance. In addition, whether COVID-19-positive patients should contribute to certain trial endpoints and dose-limiting toxicity assessment where COVID-19 is a possible cause of death or adverse events needs to be assessed, as well as how to perform sample-size calculations that account for new background rates of COVID-19-related infection, morbidity and mortality in the trial population. This is a major issue for ongoing trials, and aside from possible requirements for sample-size adjustment, imbalances between study arms in their infection rates may affect the interpretation of results. Trial-specific solutions are required, informed by policy and emerging data. In general, we would advocate a pragmatic approach, with the assumption that COVID-19 will become endemic and its associated morbidity and mortality will become new realities that must be accounted for throughout the spectrum of clinical cancer research, with protocols having inbuilt contingencies, flexibilities and appropriate sample sizes. Prioritization of primary trial objectives over other objectives will help ensure scientific integrity while still allowing researchers to learn as much as possible from each patient (as is safe and practicable). Changes and compromises made can be ‘de-escalated’ as COVID-19 recedes and depending on the availability of effective vaccination and therapeutics. The inflexibility of current protocols has been exposed by the current pandemic, and a new level of robustness is needed for resilience to COVID-19 and potential future threats.
Research studies should be prioritized for (re-)opening on the basis of risk/benefit assessments, and this should mirror the methods used for the prioritization of standard-of-care treatments1. While all phases of drug development should continue as fully as possible, where required, prioritization should be given to trials with the greatest likelihood of therapeutic success (balanced with acceptable study risks) or for which patient populations have the poorest prognosis or no standard treatment options. ‘Placebo-only’–controlled studies should generally be given lower priority. Patients must be counseled about the competing risks of COVID-19, including treatment interruptions and potential difficulties in dealing with treatment complications.
Remarkable changes in healthcare have occurred rapidly in response to the COVID-19 pandemic, many of which are positive and will be continued in the longer term. Unmitigated challenges will have a profound ‘knock-on’ effect on clinical cancer research. To find new therapies, researchers and clinicians must therefore continue to adapt to the new normal of healthcare delivery and risk management. Adopting a patient-centric view of clinical research and modernizing clinical trial protocols may enhance patient safety and experience while improving research efficiency and outcomes.
References
van de Haar, J. et al. Nat. Med. 26, 665–671 (2020).
Mann, D.M., Chen, J., Chunara, R., Testa, P.A. & Nov, O. J. Am. Med. Inform. Assoc. https://doi.org/10.1093/jamia/ocaa072 (2020).
Marandino, L., Necchi, A., Aglietta, M. & Di Maio, M. JCO Oncol. Pract. https://doi.org/10.1200/OP.20.00237 (2020).
Liao, Y., Thompson, C., Peterson, S., Mandrola, J. & Beg, M. S. Am. Soc. Clin. Oncol. Educ. Book 39, 115–121 (2019).
de Paula, B.H.R., Araújo, I., Bandeira, L., Barreto, N.M.P.B. & Doherty, G.J. Lancet Oncol. https://doi.org/10.1016/S1470-2045(20)30226-6 (2020).
Shenoy, P. Perspect. Clin. Res. 6, 173–174 (2015).
Simon, C. M., Schartz, H. A., Rosenthal, G. E., Eisenstein, E. L. & Klein, D. W. J. Empir. Res. Hum. Res. Ethics 13, 338–348 (2018).
Kim, E. S. et al. J. Clin. Oncol. 35, 3737–3744 (2017).
Gandhi, M., Yokoe, D.S. & Havlir, D.V. N. Engl. J. Med. https://doi.org/10.1056/nejme2009758 (2020).
Castelletti, S. et al. Int. J. Cardiol. 266, 89–94 (2018).
Basch, E. et al. J. Am. Med. Assoc. https://doi.org/10.1001/jama.2017.7156 (2017).
Heider, K. et al. Clin. Chem. 66, 697–705 (2020).
Denys, H. et al. Breast Cancer Res. Treat. 181, 97–105 (2020).
Tan, A. C., Ashley, D. M. & Khasraw, M. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.ccr-20-1364 (2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.J.D. reports personal fees (consulting fee or speaker honoraria) from AstraZeneca, Amgen, Boehringer Ingelheim, Bayer and Roche, outside the submitted work, and is principal or chief investigator for academic and industry-sponsored clinical trials.
Rights and permissions
About this article
Cite this article
Doherty, G.J., Goksu, M. & de Paula, B.H.R. Rethinking cancer clinical trials for COVID-19 and beyond. Nat Cancer 1, 568–572 (2020). https://doi.org/10.1038/s43018-020-0083-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-020-0083-x
This article is cited by
-
Digital health technologies and Alzheimer’s disease clinical trials: might decentralized clinical trials increase participation by people with cognitive impairment?
Alzheimer's Research & Therapy (2023)
-
Revitalising cancer clinical trials: definitely time for patient-centred reform
British Journal of Cancer (2023)
-
Oncology During the COVID-19 Pandemic: a Lockdown Perspective
Current Oncology Reports (2022)
-
Improving clinical paediatric research and learning from COVID-19: recommendations by the Conect4Children expert advice group
Pediatric Research (2022)
-
Current Challenges Faced by Cancer Clinical Trials in Addressing the Problem of Under-Representation of Older Adults: A Narrative Review
Oncology and Therapy (2021)