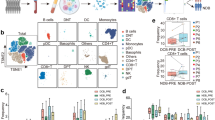
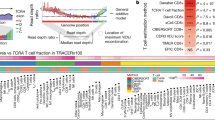
Abstract
Tumour mutational burden (TMB) predicts immunotherapy outcome in non-small cell lung cancer (NSCLC), consistent with immune recognition of tumour neoantigens. However, persistent antigen exposure is detrimental for T cell function. How TMB affects CD4 and CD8 T cell differentiation in untreated tumours and whether this affects patient outcomes is unknown. Here, we paired high-dimensional flow cytometry, exome, single-cell and bulk RNA sequencing from patients with resected, untreated NSCLC to examine these relationships. TMB was associated with compartment-wide T cell differentiation skewing, characterized by loss of TCF7-expressing progenitor-like CD4 T cells, and an increased abundance of dysfunctional CD8 and CD4 T cell subsets with strong phenotypic and transcriptional similarity to neoantigen-reactive CD8 T cells. A gene signature of redistribution from progenitor-like to dysfunctional states was associated with poor survival in lung and other cancer cohorts. Single-cell characterization of these populations informs potential strategies for therapeutic manipulation in NSCLC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The tumour region RNA-seq data, bulk RNA-seq data from sorted T cells, scRNA-seq data from sorted neoantigen-reactive T cells, TCR-seq data from sorted T cells, and flow cytometry data (in each case from the TRACERx study) used or analyzed during this study are available through the CRUK and UCL Cancer Trials Centre (ctc.tracerx@ucl.ac.uk) for non-commercial research purposes. Access will be granted upon review of a project proposal, which will be evaluated by a TRACERx data access committee, and entering into an appropriate data access agreement, subject to any applicable ethical approvals.
Code availability
Scripts to reproduce the figures can be obtained from the corresponding authors upon reasonable request.
References
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Rizvi, N. A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Mcgranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Gros, A. et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med. 22, 433–438 (2016).
Van Allen, E. M. et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211 (2015).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Thommen, D. S. & Schumacher, T. N. T cell dysfunction in cancer. Cancer Cell 33, 547–562 (2018).
Reading, J. L. et al. The function and dysfunction of memory CD8+ T cells in tumor immunity. Immunol. Rev. 283, 194–212 (2018).
Zinkernagel, R. M. et al. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol. Rev. 156, 199–209 (1997).
Rolland, M. et al. Recognition of HIV-1 peptides by host CTL is related to HIV-1 similarity to human proteins. PLoS ONE 2, e823 (2007).
Neefjes, J. & Ovaa, H. A peptide’s perspective on antigen presentation to the immune system. Nat. Chem. Biol. 9, 769–775 (2013).
Kaech, S. M. & Wherry, E. J. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27, 393–405 (2007).
Kaufmann, D. E. et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 8, 1246–1254 (2007).
Day, C. L. et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006).
Han, S., Asoyan, A., Rabenstein, H., Nakano, N. & Obst, R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc. Natl Acad. Sci. USA 107, 20453–20458 (2010).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Philip, M. & Schietinger, A. Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr. Opin. Immunol. 58, 98–103 (2019).
Kallies, A., Zehn, D. & Utzschneider, D. T. Precursor exhausted T cells: key to successful immunotherapy? Nat. Rev. Immunol. 20, 128–136 (2020).
Crawford, A. et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302 (2014).
Fletcher, J. M. et al. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J. Immunol. 175, 8218–8225 (2005).
Palmer, P. E., Boritz, E. & Wilson, C. C. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4+ T cell maturation and function. J. Immunol. 172, 3337–3347 (2004).
Patil, V. S. et al. Precursors of human CD4+ cytotoxic T lymphocytes identified by single-cell transcriptome analysis. Sci. Immunol. 3, eaan8664 (2018).
Di Mitri, D. et al. Reversible senescence in human CD4+CD45RA+CD27− memory T cells. J. Immunol. 187, 2093–2100 (2011).
Sade-Feldman, M. et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175, 998–1013.e20 (2018).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Utzschneider, D. T. et al. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427 (2016).
Siddiqui, I. et al. Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10 (2019).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Smith, C. M. et al. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nat. Immunol. 5, 1143–1148 (2004).
Matloubian, M., Concepcion, R. J. & Ahmed, R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68, 8056–8063 (1994).
Schietinger, A., Philip, M., Liu, R. B., Schreiber, K. & Schreiber, H. Bystander killing of cancer requires the cooperation of CD4+ and CD8+ T cells during the effector phase. J. Exp. Med. 207, 2469–2477 (2010).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Tran, E. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014).
Jamal-Hanjani, M. et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 376, 2109–2121 (2017).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2018).
Thommen, D. S. et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 24, 994–10014 (2018).
Brenchley, J. M. et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101, 2711–2720 (2003).
Simoni, Y. et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018).
Schietinger, A. et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 45, 389–401 (2016).
Simonetta, F. et al. High eomesodermin expression among CD57+ CD8+ T cells identifies a CD8+ T cell subset associated with viral control during chronic human immunodeficiency virus infection. J. Virol. 88, 11861–11871 (2014).
Li, H. et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell 176, 775–789.e18 (2019).
Guo, X. et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 24, 978–985 (2018).
Gros, A. et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 124, 2246–2259 (2014).
Okoye, A. et al. Progressive CD4+ central-memory T cell decline results in CD4+ effector-memory insufficiency and overt disease in chronic SIV infection. J. Exp. Med. 204, 2171–2185 (2007).
Joshi, K. et al. Spatial heterogeneity of the T cell receptor repertoire reflects the mutational landscape in lung cancer. Nat. Med. 25, 1549–1559 (2019).
Baitsch, L. et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Shin, B. et al. Effector CD4 T cells with progenitor potential mediate chronic intestinal inflammation. J. Exp. Med. 215, 1803–1812 (2018).
Tilstra, J. S. et al. Kidney-infiltrating T cells in murine lupus nephritis are metabolically and functionally exhausted. J. Clin. Invest. 128, 4884–4897 (2018).
Liston, A. et al. Inhibition of CCR6 function reduces the severity of experimental autoimmune encephalomyelitis via effects on the priming phase of the immune response. J. Immunol. 182, 3121–3130 (2009).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Hombrink, P. et al. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat. Immunol. 17, 1467–1478 (2016).
Thommen, D. S. et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol. Res. 3, 1344–1355 (2015).
Philip, M. et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456 (2017).
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 15, 1484–1506 (2020).
Xing, S. et al. Tcf1 and Lef1 transcription factors establish CD8+ T cell identity through intrinsic HDAC activity. Nat. Immunol. 17, 695–703 (2016).
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018).
Brummelman, J. et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8+ T cells infiltrating human tumors. J. Exp. Med. 215, 2520–2535 (2018).
He, Y. et al. MHC class II expression in lung cancer. Lung Cancer 112, 75–80 (2017).
Gejman, R. S. et al. Rejection of immunogenic tumor clones is limited by clonal fraction. eLife 7, e41090 (2018).
Roth, A. et al. PyClone: statistical inference of clonal population structure in cancer. Nat. Methods 11, 396–398 (2014).
Hendry, S. et al. Assessing tumor-infiltrating lymphocytes in solid tumors. Adv. Anat. Pathol. 24, 311–335 (2017).
Denkert, C. et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod. Pathol. 29, 1155–1164 (2016).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830.e14 (2018).
Liu, J. et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell 173, 400–416.e11 (2018).
Ellrott, K. et al. Scalable open science approach for mutation calling of tumor exomes using multiple genomic pipelines. Cell Syst. 6, 271–281.e7 (2018).
Nowicka, M. et al. CyTOF workflow: differential discovery in high-throughput high-dimensional cytometry datasets. F1000Res. 6, 748 (2017).
Van Gassen, S. et al. FlowSOM: using self-organizing maps for visualization and interpretation of cytometry data. Cytom. A 87, 636–645 (2015).
Ahmadzadeh, M. et al. Tumor-infiltrating human CD4+ regulatory T cells display a distinct TCR repertoire and exhibit tumor and neoantigen reactivity. Sci. Immunol. 4, eaao4310 (2019).
Lun, A. T. L., Richard, A. C. & Marioni, J. C. Testing for differential abundance in mass cytometry data. Nat. Methods 14, 707–709 (2017).
Weber, L. M. & Robinson, M. D. Comparison of clustering methods for high-dimensional single-cell flow and mass cytometry data. Cytom. A 89, 1084–1096 (2016).
Rosenthal, R. et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 567, 479–485 (2019).
Using C1 to Generate Single-Cell cDNA Libraries for mRNA Sequencing Protocol PN 100-7168 M1 (Fluidigm, 2018).
Li, W. V. & Li, J. J. An accurate and robust imputation method scImpute for single-cell RNA-Seq data. Nat. Commun. 9, 997 (2018).
Oetjen, K. A. et al. Human bone marrow assessment by single-cell RNA sequencing, mass cytometry, and flow cytometry. JCI Insight 3, 124928 (2018).
Soneson, C. & Robinson, M. D. Bias, robustness and scalability in single-cell differential expression analysis. Nat. Methods 15, 255–261 (2018).
Waugh, K. A. et al. Molecular profile of tumor-specific CD8+ T cell hypofunction in a transplantable murine cancer model. J. Immunol. 197, 1477–1488 (2016).
Godec, J. et al. Compendium of immune signatures identifies conserved and species-specific biology in response to inflammation. Immunity 44, 194–206 (2016).
Charoentong, P. et al. Pan-cancer immunogenomic analyses reveal genotype–immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 18, 248–262 (2017).
Danaher, P. et al. Gene expression markers of tumor infiltrating leukocytes. J. Immunother. Cancer 5, 18 (2017).
Aran, D., Hu, Z. & Butte, A. J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 18, 220 (2017).
Bindea, G. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013).
Zheng, C. et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell 169, 1342–1356.e16 (2017).
Acknowledgements
We thank all of the patients who participated in this study and all of the members of the TRACERx Consortium. This work was undertaken with support from the Cancer Research UK (CRUK) and University College London (UCL) Centre (C416/A18088), the CRUK Lung Cancer Centre of Excellence (C5759/A20465), a Cancer Immunotherapy Accelerator Award (CITA-CRUK; C33499/A20265), the National Institute for Health Research UCL Hospitals Biomedical Research Centre (B.C., S.A.Q. and C.S.) and the CRUK and UCL Experimental Cancer Medicine Centre. S.A.Q. received a CRUK Senior Cancer Research Fellowship (C36463/A22246) and is funded by a CRUK Biotherapeutic Program Grant (C36463/A20764), the Rosetrees and Stoneygate trusts (A1388) and a donation from the Khoo Teck Puat UK Foundation via the UCL Cancer Institute Research Trust (539288). C.S. is Royal Society Napier Research Professor. This work was supported by the Francis Crick Institute that receives its core funding from Cancer Research UK (FC001169, FC001202), the UK Medical Research Council (FC001169, FC001202), and the Wellcome Trust (FC001169, FC001202). C.S. is funded by Cancer Research UK (TRACERx, PEACE and CRUK Cancer Immunotherapy Catalyst Network), the CRUK Lung Cancer Centre of Excellence, the Rosetrees Trust, NovoNordisk Foundation (ID16584) and the Breast Cancer Research Foundation (BCRF). This research is supported by a Stand Up To Cancer‐LUNGevity-American Lung Association Lung Cancer Interception Dream Team Translational Research Grant (Grant Number: SU2C-AACR-DT23-17). Stand Up To Cancer is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the Scientific Partner of SU2C. CS receives funding from the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP7/2007-2013) Consolidator Grant (FP7-THESEUS-617844), European Commission ITN (FP7-PloidyNet 607722), an ERC Advanced Grant (PROTEUS) from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 835297), and Chromavision from the European Union’s Horizon 2020 research and innovation programme (grant agreement 665233). B.C. is supported by a CRUK Project Grant. K.S.P. receives funding from the NIHR BTRU in Stem Cells and Immunotherapies (167097), of which he is the Scientific Director. E.G. is funded by a Wellcome Trust Research Training Fellowship. S.R.H. is funded by a grant from the European Research Council, StG 677268 NextDART. The TRACERx study (Clinicaltrials.gov number NCT01888601) is sponsored by UCL. We thank the UCL Cancer Institute Flow Cytometry Translational Technology Platform—in particular Y. Guo, G. Morrow and B. Wilbourn—for support and instrumentation.
Author information
Authors and Affiliations
Consortia
Contributions
S.A.Q., C.S. and J.L.R. supervised the project. J.L.R., S.A.Q. and C.S. conceived of and designed the project. E.G. designed and carried out the bioinformatics analyses. J.L.R., J.Y.H. and E.G. designed, carried out and interpreted the wet laboratory experiments. E.G., J.L.R., S.A.Q. and C.S. wrote the manuscript. M.R.d.M., R.R., N.J.B., G.A.W., K.L., L.C., J.A.G.-A., K.B., D.B., Y.S. and K.A. contributed to data processing and analyses. V.T., supervised by T.E., carried out the RNA-seq experiments. K.J., A.J.S.F., A.B.A., A.G., M.W.S., Y.N.S.W., M.V.D.M., W.D., F.G.-C., P.D.B. and T.O. contributed to the wet laboratory experiments. S.K.S. and S.R., supervised by S.R.H., carried out the multimer reactivity screens. I.U., M.I., T.R. and A.W., supervised by B.C., carried out the TCR-seq. M.J.-H., S.V., C.S., A.H. and the TRACERx Consortium coordinated the clinical trials and provided patient samples and patient data. R.S., T.L., M.A.B., D.A.M., C.T.H. and S.L. carried out the pathology TIL estimates. S.A.Q., C.S., B.C., N.M., K.S.P., Y.Y., J.H. and S.T. contributed to project management and supervision, as well as providing valuable critical discussion.
Corresponding authors
Ethics declarations
Competing interests
S.A.Q., K.S.P. and C.S. are co-founders of Achilles Therapeutics. C.S. receives grant support from Pfizer, AstraZeneca, BMS, Roche–Ventana and Boehringer Ingelheim. C.S. has consulted for Pfizer, Novartis, GlaxoSmithKline, MSD, BMS, Celgene, AstraZeneca, Illumina, Genentech, Roche–Ventana, GRAIL, Medicxi and the Sarah Cannon Research Institute. C.S. is a shareholder of ApoGen Biotechnologies, Epic Bioscience and GRAIL, and has stock options in and is co-founder of Achilles Therapeutics. R.R., N.M. and G.A.W. have stock options in and have consulted for Achilles Therapeutics. J.L.R. and M.A.B. have consulted for Achilles Therapeutics. P.D.B., T.O. and M.W.S. are employees of Achilles Therapeutics.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Sample data availability.
(a) Sample data availability and disposition for TRACERx 100 flow cytometry and RNA sequencing cohorts, with details of matched data relevant to key analyses. * 41 regions had >2000 live CD3+ events; ^ 18 NTA specimens in total, including from two patients without matched tumour tissue; # 37 regions had WES data and >2000 live CD3+ events. (b) Patients and regional data availability for flow cytometry cohorts 1 and 2. All patients with at least one tumour region are shown.
Extended Data Fig. 2 Progenitor-like and dysfunctional T cell subsets correlate with clonal mutational burden and their abundance associates with patient outcomes.
(a) Gating strategy to define CD4 Early, Tdys and TDT populations. (b) For n=20 lung samples with distinct CD4 staining, the percentage of CD4+ cells amongst manually gated Early, Tdys and TDT populations from n=61 total samples, is shown for each subset. (c) Gating strategy to define CD8 Naive-like, Tdys and TDT populations. (d) Boxplots show the Spearman correlation between cluster abundance and TMB (n=39 regions from 15 patients) across all iterations (n=1000) of the clustering workflow. Each point represents the result of a single run. (e) Heatmap showing cluster stability across 1000 clustering iterations. The cluster identity of each cell was determined for one representative iteration (labels are on the left of the heatmap). For each cell, the probability of being assigned to each cluster (labelled below the plot) across all iterations is represented. (f) Relationship between CD4 population abundance (60 regions from 29 patients) and tumour genomic features. Two-sided p-values and regression slopes (β coefficients) reflecting the direction and magnitude of relationships tested are from linear mixed effects regression models accounting for tumour histology and multiregionality. (g, h) Percentage of cells amongst manually gated cohort 2 CD8 (g) and CD4 (h) populations positive for key markers (26 regions from 16 patients). All comparisons p<0.05 by two-sided Wilcoxon rank sum test except for those labelled. Violin plots show median and interquartile range. (i) Neoantigen-multimer reactive (Mult+) CD8 T cell identification and PD1 expression for two patients in comparison to matched multimer non-reactive (Mult-), NTA and circulating (PBMC) CD8 T cells. Line graph shows CD8 T cell PD1 MFI (relative to PBMC) in Mult+, Mult- and NTA populations. Data points show mean PD1 MFI from n=4 multimer reactive populations from n=3 patients, error bars show SEM. P-values are from paired 2-Way ANOVA (Fisher’s least significant difference test). (j) Disease free survival (DFS) probability of patients with high vs. low abundance of CD4 (upper row) and CD8 subsets (from n=29 and n=31 patients respectively), categorized according to the median value. The number of patients at risk at each time point, log-rank p-value and hazard ratios with 95% confidence intervals are shown. (k) Sort strategy for CD4 (top) and CD8 subsets, for TCRseq. (l) Venn diagrams show CDR3 beta chain sharing between CD4 (left two diagrams) and CD8 subsets, for two patients each. Boxplots in (b) and (d) represent median and interquartile range.
Extended Data Fig. 3 Identification and single cell transcriptomic characterization of progenitor-like and dysfunctional T cell subsets.
(a) Full gating strategy to identify the CD4 Early, CD8 Naïve-like, CD8 Tdys and CD8 TDT subsets by single T cell RNA expression. (b, c) Confirmation of CD4 (n=590 cells; b) and CD8 (n=206 cells; c) subset identity by evaluating expression of genes not used in the gating strategy but whose relative expression is known based on analysis of flow cytometry data. Each point represents an individual T cell and two-sided Wilcoxon rank sum test p-values are shown (***p<0.0001). Violin plots show the median and interquartile range. (d, e) GSEA to evaluate enrichment of gene sets upregulated in published T cell dysfunction datasets (d) and sorted CD8 Tdys and multimer reactive cells (e), amongst genes ranked by their expression in CD4 TDT vs. Early (143 vs. 175 cells) and CD8 TDT vs Naive-like populations (143 vs. 19 cells). For each gene set tested, the top 200 most differentially expressed genes were selected. Normalized enrichment scores (NES) and FDR adjusted p-values from permutation tests are shown. (f) GSEA to confirm the T central memory like transcriptional status of CD4 Early vs. Tdys/TDT subsets (175 vs. 415 cells). Normalized enrichment scores (NES) and FDR adjusted p-values by permutation test are shown.
Extended Data Fig. 4 Expression profile of progenitor-like and dysfunctional T cell subsets.
(a) Differentially expressed transcription factor encoding genes in CD4 (n=590 cells) and CD8 (n=206 cells) subsets at the single T cell RNA expression level. Each gene has >2-fold differential expression in one subset with FDR adjusted p<0.05 (quasi-likelihood F-test with edgeR). Differentially expressed genes encoding adhesion molecules and chemokine receptors (b) and ITIM containing proteins (c); All genes shown are >2-fold differentially expressed between subsets within the same compartment, FDR adjusted p<0.05. (d) Expression of the top 500 most variably expressed genes between CD4 and CD8 subsets.
Extended Data Fig. 5 TCF7 and CD39 protein expression in CD4 and CD8 T cell subsets.
(a) Flow cytometry of concatenated data from n=3 patients (CRUK0939, CRUK0952 and CRUK1037) in manually-gated subsets of tumour infiltrating CD4 (Early; CD45RA-PD1-FOXP3-CD27+CCR7+, Tdys; FOXP3-CD27+PD1hiCD57-, TDT FOXP3-CD27+PD1hiCD57+) and CD8 (Naïve-like; CD45RA+PD1-CD27+CD57-, Tdys; CD45RA-CD27+PD1hiCD57-, TDT CD45RA-CD27+PD1hiCD57+) T cells. (b) Quantification of TCF7 and CD39 expression in CD8 (top row) and CD4 subsets identified amongst n=3 patients in (a). Error bars represent the SEM. (c) PD1 vs. TCF7 expression of CD4 and CD8 TILs from the same patients as (a).
Extended Data Fig. 6 Transcriptional similarity and gene pathway analysis amongst dysfunctional subsets.
(a) For each gene set tested in enrichment analysis, leading edge genes shared between at least two sets were identified and their overlap between CD4 and CD8 dysfunctional population is shown. (b) Of the 19 shared leading edge genes common to both CD4 and CD8 populations, 17 were expressed in single cell RNA sequencing data from multimer reactive cells. Violin plots show expression in multimer positive (n=36) vs. negative cells (n=39). Unadjusted two-sided Wilcoxon rank sum test p-values are shown. Violin plots represent the median and interquartile range. (c) Bar chart shows enrichment in multimer reactive vs. non-reactive cells of shared GO terms that distinguish dysfunctional T cell populations, identified in Fig. 3E. Selected pathways are identified in the table and their enrichment within each population vs. control is shown in (d). FDR adjusted two-sided Wilcoxon rank sum test p-values are represented. CD8 Tdys vs. Naive-like (143 vs. 19 cells), CD4 Tdys vs. Early (272 vs. 175 cells), CD4 TDT vs. Early (143 vs. 175 cells) and Mult+ vs. Mult- (36 vs. 39 cells).
Extended Data Fig. 7 Transcriptional evidence of signalling pathways active between T cell subsets.
(a) Network diagram of ligand–receptor interactions as determined by cellPhoneDB; Solid lines represent pathways between two populations, the width of each line is proportional to the number of pathways. For each pair of populations, pathways were split depending on which population is ligand-bearing vs. receptor-bearing. Arrows indicate communication from ligand-bearing to receptor-bearing populations. (b) Summary of overlap in reciprocal pathways between population pairs. The heatmap represents the Jaccard similarity index of overlapping pathways for each pair of populations. (c) Summary of directed pathway counts. The heatmap represents the number of pathways for each directed pair of populations. (d) Number of pathways where each population is the ligand-bearing partner (left column) or receptor-bearing partner (right column) and the ratio between the count of each group. (e) Summary of ligand–receptor interactions. Log2 means of the average expression level of receptor-ligand pair genes are shown.
Extended Data Fig. 8 Pan-TCGA association between a signature of T cell differentiation skewing and patient outcomes.
(a) Forest plot showing the relationship between the TL-DS signature and survival across TCGA cohorts (n=6853 patients). HRs and FDR adjusted p-values are from univariable Cox regression analysis. (b) Relationship between the TL-DS signature and survival, corrected for T cell infiltration, TMB and stage, in cohorts from (b) in which the signature predicted survival (9 cohorts, n=2418 patients). HRs and p-values are from multivariable Cox regression analysis. Cohorts in which the relationship was significant are shown.
Extended Data Fig. 9 Single T cell RNAseq cluster analysis.
(a, b) UMAP dimension reduction plot of NSCLC CD4 (a) and CD8 (b) TIL single cell RNA sequencing data (2469 and 1508 cells respectively). Manually identified subsets are located in the upper panels of A and B. Clustering analysis reveals 10 CD4 and 10 CD8 subsets (lower panels). Relative expression (z-score) of selected genes is shown in the adjacent heatmaps (each column represents a single cell).
Extended Data Fig. 10 Marker and transcriptional changes within the CD4 Early population in relation to TMB and subset abundance.
(a) Workflow to determine the relationship between flow cytometry measured marker expression intensity and TMB in CD4 Early (n=23,597 cells), Tdys (n=25,271 cells) and TDT (n=11,880 cells) subsets. Each point represents an individual cell, FDR adjusted two-sided p-values and regression coefficients were derived from linear mixed effects models accounting for patient histology and tumour multiregionality and plotted in (b). Volcano plots show the relationship between marker intensity and TMB for each CD4 subset. (c) Change in CD4 Tdys and TDT with Early abundance (as a percentage of all CD4+ cells, n=12 patients). Two-sided Pearson p- and r-values are shown. Shaded bands represents the 95% confidence interval of a linear regression slope. (d) GSEA of T helper subset signatures enriched in Tdys and TDT vs. Early (n=590 cells), using modules from Charoentong et al. 201779. Normalized enrichment scores (NES) and FDR adjusted p-values from permutation tests are shown. (e) Correlation between falling abundance of the CD4 Early population (175 cells, n=12 patients) and expression of gene signatures from (d) indicative of CD4 later differentiation state. Two-sided p-values and regression coefficients were derived from linear mixed effects models with patient as the random effect. Published T cell subset signatures used in the study are summarized in (f).
Supplementary information
Rights and permissions
About this article
Cite this article
Ghorani, E., Reading, J.L., Henry, J.Y. et al. The T cell differentiation landscape is shaped by tumour mutations in lung cancer. Nat Cancer 1, 546–561 (2020). https://doi.org/10.1038/s43018-020-0066-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-020-0066-y
This article is cited by
-
A comprehensive single-cell map of T cell exhaustion-associated immune environments in human breast cancer
Nature Communications (2023)
-
Stem-like exhausted and memory CD8+ T cells in cancer
Nature Reviews Cancer (2023)
-
Pan-cancer T cell atlas links a cellular stress response state to immunotherapy resistance
Nature Medicine (2023)
-
Deciphering the immunopeptidome in vivo reveals new tumour antigens
Nature (2022)
-
Tumour burden and efficacy of immune-checkpoint inhibitors
Nature Reviews Clinical Oncology (2022)