Abstract
Advanced and metastatic tumors with complex treatment histories drive cancer mortality. Here we describe the POG570 cohort, a comprehensive whole-genome, transcriptome and clinical dataset, amenable for exploration of the impacts of therapies on genomic landscapes. Previous exposure to DNA-damaging chemotherapies and mutations affecting DNA repair genes, including POLQ and genes encoding Polζ, were associated with genome-wide, therapy-induced mutagenesis. Exposure to platinum therapies coincided with signatures SBS31 and DSB5 and, when combined with DNA synthesis inhibitors, signature SBS17b. Alterations in ESR1, EGFR, CTNNB1, FGFR1, VEGFA and DPYD were consistent with drug resistance and sensitivity. Recurrent noncoding events were found in regulatory region hotspots of genes including TERT, PLEKHS1, AP2A1 and ADGRG6. Mutation burden and immune signatures corresponded with overall survival and response to immunotherapy. Our data offer a rich resource for investigation of advanced cancers and interpretation of whole-genome and transcriptome sequencing in the context of a cancer clinic.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Genomic and transcriptomic sequence datasets, including metadata with library construction and sequencing approaches have been deposited at the European Genome–phenome Archive (EGA, http://www.ebi.ac.uk/ega/) as part of the study EGAS00001001159 with accession numbers as listed in Supplementary Table 1. Data on mutations, copy changes and expression from tumor samples in the POG program organized by OncoTree classification (http://oncotree.mskcc.org) are also accessible from https://www.personalizedoncogenomics.org/cbioportal/. The complete small mutation catalog and gene expression TPMs are available for download from http://bcgsc.ca/downloads/POG570/. Previously published TCGA and PCAWG data that were re-analyzed here are available from data portals (https://portal.gdc.cancer.gov/ and https://dcc.icgc.org/) with sample barcodes as listed in Supplementary Table 9. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability
The bioinformatics analyses were performed using open-source software, including Burrows–Wheeler alignment tool (v.0.5.7 for up to 125 bp reads and v.0.7.6a for 150 bp reads), CNAseq91 (v.0.0.6), APOLLOH92 (v.0.1.1), SAMtools93 (v.0.1.17), MutationSeq94 (v.1.0.2 and v.4.3.5), Strelka95 (v.1.0.6), SNPEff96 (v.3.2 for somatic and v.4.1 for germline), ABySS97 (v.1.3.4), TransABySS97,98 (v.1.4.10), Chimerascan99 (v.0.4.5), DeFuse100 (v.0.6.2), Manta101 (v.1.0.0), Delly102 (v.0.7.3), MAVIS71 (v.2.1.1), STAR73 (v.2.5.2b), RSEM74 (v.1.3.0), MSIsensor76 (v.0.2), HRDtools16 (v.0.0.0.9), BioBloomTools78 (v.2.0.11b), EXPANDS84 (v.2.1.1), SignIT (https://github.com/eyzhao/SignIT), samtools93 (v.0.1.17), ClinVar103 (v.20180905), InterVar88, ControlFREEC104 (v.5), CIBERSORT89 (v.1.04), Jaguar105 (v.2.0.3), MiXCR90 (Java, v.2.1.2) and VDJtools106 (v.1.1.9). Additional packages used for meta-analyses include R packages ClusteredMutations (v.1.0.1), vegan (v.2.5.3), ConsensusClusterPlus (v.1.44.0), ComplexHeatmap (v.1.18.1), survival (v.2.42.3), survminer (v.0.4.2) and Python package scikit-learn86 (Python, v.0.20). Additional processing involved in-house scripts that are available upon request.
References
Schwaederle, M. et al. Impact of precision medicine in diverse cancers: a meta-analysis of phase II clinical trials. J. Clin. Oncol. 33, 3817–3825 (2015).
Bailey, M. H. et al. Comprehensive characterization of cancer driver genes and mutations. Cell 174, 1034–1035 (2018).
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23, 703–713 (2017).
Chalmers, Z. R. et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 9, 34 (2017).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Nik-Zainal, S. et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534, 47–54 (2016).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019).
Priestley, P. et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature 575, 210–216 (2019).
Robinson, D. R. et al. Integrative clinical genomics of metastatic cancer. Nature 548, 297–303 (2017).
van der Wekken, A. J. et al. Overall survival in EGFR mutated non-small cell lung cancer patients treated with afatinib after EGFR TKI and resistant mechanisms upon disease progression. PLoS ONE 12, e0182885 (2017).
Jeselsohn, R., De Angelis, C., Brown, M. & Schiff, R. The evolving role of the estrogen receptor mutations in endocrine therapy-resistant breast cancer. Curr. Oncol. Rep. 19, 35 (2017).
Szikriszt, B. et al. A comprehensive survey of the mutagenic impact of common cancer cytotoxics. Genome Biol. 17, 99 (2016).
Boot, A. et al. In-depth characterization of the cisplatin mutational signature in human cell lines and in esophageal and liver tumors. Genome Res. 28, 654–665 (2018).
Murugaesu, N. et al. Tracking the genomic evolution of esophageal adenocarcinoma through neoadjuvant chemotherapy. Cancer Discov. 5, 821–831 (2015).
Cristescu, R. et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362, eaar3593 (2018).
Zhao, E. Y. et al. Homologous recombination deficiency and platinum-based therapy outcomes in advanced breast cancer. Clin. Cancer Res. 23, 7521–7530 (2017).
Laskin, J. et al. Lessons learned from the application of whole-genome analysis to the treatment of patients with advanced cancers. Cold Spring Harb. Mol. Case Stud. 1, a000570 (2015).
Majounie, E. et al. Fluorouracil sensitivity in a head and neck squamous cell carcinoma with a somatic DPYD structural variant. Cold Spring Harb. Mol. Case Stud. 6, a004713 (2019).
Jones, M. R. et al. NRG1 gene fusions are recurrent, clinically actionable gene rearrangements in KRAS wild-type pancreatic ductal adenocarcinoma. Clin. Cancer Res. 25, 4674–4681 (2019).
Jones, M. R. et al. Response to angiotensin blockade with irbesartan in a patient with metastatic colorectal cancer. Ann. Oncol. 27, 801–806 (2016).
Thibodeau, M. L. et al. Whole genome and whole transcriptome genomic profiling of a metastatic eccrine porocarcinoma. NPJ Precis. Oncol. 2, 8 (2018).
Chooback, N. et al. Carcinoma ex pleomorphic adenoma: case report and options for systemic therapy. Curr. Oncol. 24, e251–e254 (2017).
Bose, P. et al. Integrative genomic analysis of ghost cell odontogenic carcinoma. Oral Oncol. 51, e71–e75 (2015).
Cancer Genome Atlas Research Network. Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell 171, 950–965 (2017).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Davare, M. A. et al. Rare but recurrent ROS1 fusions resulting from chromosome 6q22 microdeletions are targetable oncogenes in glioma. Clin. Cancer Res. 24, 6471–6482 (2018).
Tozbikian, G. H. & Zynger, D. L. A combination of GATA3 and SOX10 is useful for the diagnosis of metastatic triple-negative breast cancer. Hum. Pathol. 85, 221–227 (2019).
Harbhajanka, A. et al. Clinicopathological, immunohistochemical and molecular correlation of neural crest transcription factor SOX10 expression in triple-negative breast carcinoma. Hum. Pathol. 80, 163–169 (2018).
Angus, L. et al. The genomic landscape of metastatic breast cancer highlights changes in mutation and signature frequencies. Nat. Genet. 51, 1450–1458 (2019).
Drago, J. Z. et al. FGFR1 amplification mediates endocrine resistance but retains TORC sensitivity in metastatic hormone receptor-positive (HR+) breast cancer. Clin. Cancer Res. 25, 6443–6451 (2019).
Sokol, E. S. et al. Loss of function of NF1 is a mechanism of acquired resistance to endocrine therapy in lobular breast cancer. Ann. Oncol. 30, 115–123 (2019).
Hu, J., Adar, S., Selby, C. P., Lieb, J. D. & Sancar, A. Genome-wide analysis of human global and transcription-coupled excision repair of UV damage at single-nucleotide resolution. Genes Dev. 29, 948–960 (2015).
Pleasance, E. D. et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 463, 184–190 (2010).
Huang, F. W. et al. Highly recurrent TERT promoter mutations in human melanoma. Science 339, 957–959 (2013).
Horn, S. et al. TERT promoter mutations in familial and sporadic melanoma. Science 339, 959–961 (2013).
Weinhold, N., Jacobsen, A., Schultz, N., Sander, C. & Lee, W. Genome-wide analysis of noncoding regulatory mutations in cancer. Nat. Genet. 46, 1160–1165 (2014).
Wu, S. et al. Whole-genome sequencing identifies ADGRG6 enhancer mutations and FRS2 duplications as angiogenesis-related drivers in bladder cancer. Nat. Commun. 10, 720 (2019).
Dentro S. C. et al. Portraits of genetic intra-tumour heterogeneity and subclonal selection across cancer types. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/312041v4.
Arasada, R. R. et al. Notch3-dependent β-catenin signaling mediates EGFR TKI drug persistence in EGFR-mutant NSCLC. Nat. Commun. 9, 3198 (2018).
Howie, L. J. et al. FDA approval summary: pertuzumab for adjuvant treatment of HER2-positive early breast cancer. Clin. Cancer Res. 25, 2949–2955 (2019).
Matsusaka, S. & Lenz, H.-J. Pharmacogenomics of fluorouracil-based chemotherapy toxicity. Expert Opin. Drug Metab. Toxicol. 11, 811–821 (2015).
Letouzé, E. et al. Mutational signatures reveal the dynamic interplay of risk factors and cellular processes during liver tumorigenesis. Nat. Commun. 8, 1315 (2017).
McGranahan, N. et al. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci. Transl. Med. 7, 283ra54 (2015).
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020).
Davies, H. et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat. Med. 23, 517–525 (2017).
Alexandrov, L. B., Nik-Zainal, S., Siu, H. C., Leung, S. Y. & Stratton, M. R. A mutational signature in gastric cancer suggests therapeutic strategies. Nat. Commun. 6, 8683 (2015).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Grolleman, J. E. et al. Mutational signature analysis reveals NTHL1 deficiency to cause a multi-tumor phenotype. Cancer Cell 35, 256–266 (2019).
Viel, A. et al. A specific mutational signature associated with DNA 8-oxoguanine persistence in MUTYH-defective colorectal cancer. EBioMedicine 20, 39–49 (2017).
Kucab, J. E. et al. A compendium of mutational signatures of environmental agents. Cell 177, 821–836.e16 (2019).
Zou, X. et al. Validating the concept of mutational signatures with isogenic cell models. Nat. Commun. 9, 1744 (2018).
Baretti, M. & Le, D. T. DNA mismatch repair in cancer. Pharmacol. Ther. 189, 45–62 (2018).
Denver, D. R., Feinberg, S., Steding, C., Durbin, M. D. & Lynch, M. The relative roles of three DNA repair pathways in preventing Caenorhabditis elegans mutation accumulation. Genetics 174, 57–65 (2006).
Telli, M. L. et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin. Cancer Res. 22, 3764–3773 (2016).
Pich, O. et al. The mutational footprints of cancer therapies. Nat. Genet. 51, 1732–1740 (2019).
Taylor, B. J. et al. DNA deaminases induce break-associated mutation showers with implication of APOBEC3B and 3A in breast cancer kataegis. eLife 2, e00534 (2013).
Lee, Y.-S., Gregory, M. T. & Yang, W. Human Pol ζ purified with accessory subunits is active in translesion DNA synthesis and complements Pol η in cisplatin bypass. Proc. Natl Acad. Sci. USA 111, 2954–2959 (2014).
McHugh, P. J., Spanswick, V. J. & Hartley, J. A. Repair of DNA interstrand crosslinks: molecular mechanisms and clinical relevance. Lancet Oncol. 2, 483–490 (2001).
Behjati, S. et al. Mutational signatures of ionizing radiation in second malignancies. Nat. Commun. 7, 12605 (2016).
Law, E. K. et al. The DNA cytosine deaminase APOBEC3B promotes tamoxifen resistance in ER-positive breast cancer. Sci. Adv. 2, e1601737 (2016).
Schrader, K. A. et al. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol. 2, 104–111 (2016).
Wong, H.-L. et al. Molecular characterization of metastatic pancreatic neuroendocrine tumors (PNETs) using whole-genome and transcriptome sequencing. Cold Spring Harb. Mol. Case Stud. 4, a002329 (2018).
Thibodeau, M. L. et al. Base excision repair deficiency signatures implicate germline and somatic MUTYH aberrations in pancreatic ductal adenocarcinoma and breast cancer oncogenesis. Cold Spring Harb. Mol. Case Stud. 5, a003681 (2019).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830 (2018).
Dieu-Nosjean, M.-C. et al. Long-term survival for patients with non-small cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 26, 4410–4417 (2008).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Topalian, S. L., Taube, J. M., Anders, R. A. & Pardoll, D. M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 16, 275–287 (2016).
Wang, J. et al. Clonal evolution of glioblastoma under therapy. Nat. Genet. 48, 768–776 (2016).
Zhou, C. & Zhang, J. Immunotherapy-based combination strategies for treatment of gastrointestinal cancers: current status and future prospects. Front. Med. 13, 12–23 (2019).
Grewal, J. K. et al. Detection and genomic characterization of a mammary-like adenocarcinoma. Cold Spring Harb. Mol. Case Stud. 3, a002170 (2017).
Reisle, C. et al. MAVIS: merging, annotation, validation, and illustration of structural variants. Bioinformatics 35, 515–517 (2019).
Chakravarty, D. et al. OncoKB: a precision oncology knowledge base. JCO Precis. Oncol. https://doi.org/10.1200/PO.17.00011 (2017).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinf. 12, 323 (2011).
Zerbino, D. R. et al. Ensembl 2018. Nucleic Acids Res. 46, D754–D761 (2018).
Niu, B. et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics 30, 1015–1016 (2014).
Timms, K. M. et al. Association of BRCA1/2defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res. 16, 475 (2014).
Chu, J. et al. BioBloom tools: fast, accurate and memory-efficient host species sequence screening using bloom filters. Bioinformatics 30, 3402–3404 (2014).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer genes. Nature 499, 214–218 (2013).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B 57, 289–300 (1995).
Alexandrov, L. B., Nik-Zainal, S., Wedge, D. C., Campbell, P. J. & Stratton, M. R. Deciphering signatures of mutational processes operative in human cancer. Cell Rep. 3, 246–259 (2013).
Fishilevich, S. et al. GeneHancer: genome-wide integration of enhancers and target genes in GeneCards. Database https://doi.org/10.1093/database/bax028 (2017).
Agarwal, V. et al. Predicting effective microRNA target sites in mammalian mRNAs. eLife 4, e05005 (2015).
Andor, N., Harness, J. V., Müller, S., Mewes, H. W. & Petritsch, C. EXPANDS: expanding ploidy and allele frequency on nested subpopulations. Bioinformatics 30, 50–60 (2014).
Flicek, P. et al. Ensembl 2014. Nucleic Acids Res. 42, D749–D755 (2014).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn Res. 12, 2825–2830 (2011).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Li, Q. & Wang, K. InterVar: clinical interpretation of genetic variants by the 2015 ACMG-AMP guidelines. Am. J. Hum. Genet. 100, 267–280 (2017).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457 (2015).
Bolotin, D. A. et al. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods 12, 380–381 (2015).
Jones, S. J. et al. Evolution of an adenocarcinoma in response to selection by targeted kinase inhibitors. Genome Biol. 11, R82 (2010).
Ha, G. et al. Integrative analysis of genome-wide loss of heterozygosity and monoallelic expression at nucleotide resolution reveals disrupted pathways in triple-negative breast cancer. Genome Res. 22, 1995–2007 (2012).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Ding, J. et al. Feature-based classifiers for somatic mutation detection in tumour-normal paired sequencing data. Bioinformatics 28, 167–175 (2012).
Saunders, C. T. et al. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 28, 1811–1817 (2012).
Cingolani, P. et al. A program for annotating and predicting the effects of single-nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6, 80–92 (2012).
Simpson, J. T. et al. ABySS: a parallel assembler for short read sequence data. Genome Res. 19, 1117–1123 (2009).
Birol, I. et al. De novo transcriptome assembly with ABySS. Bioinformatics 25, 2872–2877 (2009).
Iyer, M. K., Chinnaiyan, A. M. & Maher, C. A. ChimeraScan: a tool for identifying chimeric transcription in sequencing data. Bioinformatics 27, 2903–2904 (2011).
McPherson, A. et al. deFuse: an algorithm for gene fusion discovery in tumor RNA-seq data. PLoS Comput. Biol. 7, e1001138 (2011).
Chen, X. et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016).
Rausch, T. et al. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 28, i333–i339 (2012).
Landrum, M. J. et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 44, D862–D868 (2016).
Boeva, V. et al. Control-FREEC: a tool for assessing copy number and allelic content using next-generation sequencing data. Bioinformatics 28, 423–425 (2012).
Butterfield, Y. S. et al. JAGuaR: junction alignments to genome for RNA-seq reads. PLoS ONE 9, e102398 (2014).
Shugay, M. et al. VDJtools: unifying post-analysis of T-cell receptor repertoires. PLoS Comput. Biol. 11, e1004503 (2015).
Acknowledgements
This work would not be possible without the participation of our patients and families, the POG team, Canada’s Michael Smith Genome Sciences Centre technical platforms, the generous support of the BC Cancer Foundation and their donors and Genome British Columbia (project B20POG). We acknowledge contributions from Genome Canada and Genome BC (projects 202SEQ M.A.M. and S.M.J., 212SEQ M.A.M. and S.M.J., 12002 GBC M.A.M., S.M.J. and J.L.), Canada Foundation for Innovation (projects 20070 M.A.M. and S.M.J., 30981 M.A.M., S.M.J. and J.L., 30198 M.A.M., 33408 M.A.M. and S.M.J.) including the CGEn platform (35444 S.M.J.) and the BC Knowledge Development Fund. We acknowledge the generous support of the Canadian Institutes of Health Research Foundation Grants program (FDN 143288, M.A.M.), University of British Columbia Clinician Investigator Program (M.L.T.) and the Canadian Institutes of Health Research Vanier Canada Graduate Scholarship (E.Y.Z.). The results published here are in part based upon analyses of data generated by the following projects and obtained from dbGaP (http://www.ncbi.nlm.nih.gov/gap): TCGA managed by the National Cancer Institute and National Human Genome Research Institute (http://cancergenome.nih.gov); and the Genotype-Tissue Expression (GTEx) Project, supported by the Common Fund of the Office of the Director of the National Institutes of Health (https://commonfund.nih.gov/GTEx). Data from PCAWG managed by the International Cancer Genome Consortium was retrieved from https://dcc.icgc.org/pcawg.
Author information
Authors and Affiliations
Contributions
M.A.M., J.L., M.R.J., Y.S. and E.P. conceptualized the study. C.R., E.Y.Z., K.L.M., E.C., A.D., M.W., S.K.C., S.Z., S.B., A.M., D.D., R.D.C., D.M., M.C., C.C., D.B., S. Sadeghi, W.Z., T.W., D.C, Y.M. and S.D.B. contributed to software development and implementation. Analyses were performed by E.T., E.P., L.W., E.Y.Z., H.K., K.F., R.B., K.D., L.C., J.K.G., J.A., K.W., C.J.G., M.L.T., M.R.J., Z.B., H.P., T.V. and R.S. Data were collected and experiments were performed by A.J.M., R.A.M., Y.Z., M.R.J., Y.S., M.B., G.A.T., E.M., V.C., K.S., S.Y., D.A.R., D.W. and R.A.H. Provision of patient samples and curation of data was conducted by K.G., A.T., S. Sun, H.L., D.J.R., S.C., D.F.S., J.L., M.K.C.L., J.M.L., B.D., A. Fisic, J.N. and S.M. The original draft was written by E.P., E.T., L.W., E.Y.Z., H.K., K.D., K.W., M.R.J. and Y.S. M.A.M., J.L. and S.J.M.J. reviewed and edited the manuscript. Data visualization was conducted by E.T., H.K., R.B., E.Y.Z., L.C., K.D., E.P., K.W., K.F., Z.B. and J.K.G. Project management and co-ordination was performed by J.N., A. Fok and J.M.K. Funding was acquired by M.A.M., J.L. and S.J.M.J.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cohort demographics.
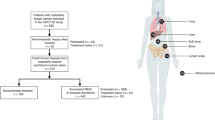
a, Drug class and frequency of prior treatment across the POG570 cohort. Drugs are grouped into classes based on mechanism (see Supplementary Table 2). b, Drug co-occurrence by patient in SARC, PANC, OV and LUNG tumor types. Darker circles indicate drugs used more frequently, and darker lines show drugs more frequently used in combination.
Extended Data Fig. 2 Genomic alterations and mutations in advanced and metastatic cancers.
a, Small mutation rates, measured in mutations/Mb, in exonic and intronic regions in comparison to intergenic regions for the largest tumor groups in the cohort. Both boxplots for each tumor type have the same n value. b, Genomic location and frequency of mutation clusters found in at least five patients across the cohort. c, Proportion of mutations that are subclonal for individual samples by tumor type (see Methods). d, Comparison of heterogeneity for subpopulation detection between EXPANDS runs of differing inputs: 100 iterations of a fixed input of 1000 mutations, and a variable input (up to a maximum of 8000, see Methods). e, Correlation of heterogeneity between EXPANDS runs of differing inputs. R and P were calculated using a Spearman correlation (n=501). f, Comparison of heterogeneity between samples with a high (>=20%) and low (<20%) proportion of subclonal mutations. The P value was calculated using a Wilcoxon rank sum test. g, Size (frequency) of subpopulations containing driver genes (see Methods) across the largest tumor types in the POG570 cohort. Higher frequencies refer to more dominant, clonal sub-populations. h, Cox proportional hazards model demonstrating the influence of tumor type, mutation burden and heterogeneity of subpopulations on overall survival from advanced disease diagnosis for all patients (n=570). n values for individual groups are indicated by the N column. Horizontal lines indicate the 95% confidence interval. The central line on the violin plots in c-d represent the median and the tips extend to the minimum and maximum values of the distribution. Boxplots in a, d and f represent the median, upper and lower quartiles of the distribution, and whiskers represent the limits of the distribution (1.5 * interquartile range). Heterogeneity is defined as the Shannon Index for the sample (see Methods). All statistical tests are two-sided. In all instances, n=number of patients in each corresponding group.
Extended Data Fig. 3 Treatment-associated recurrent mutations.
a, Frequency and occurrence of mutations in breast and lung cancer patients treated with therapies described in Fig. 3c. b, tSNE of site of origin genes (see Methods) demonstrates that gene expression separates samples by tumor type within the POG570 cohort (n=570 patients, all samples).
Extended Data Fig. 4 De novo mutation signature cosine similarities.
a-c, Cosine similarities for all de novo SBS (c), Indel (d) and DBS (e) signatures detected in the POG570 cohort with COSMIC signatures.
Extended Data Fig. 5 Additional mutation signatures.
a-b, Additional SBS (a) and indel (b) signature patterns detected in the POG570 cohort that do not strongly match any COSMIC signatures.
Extended Data Fig. 6 Mutation signature spearman correlation.
a, Pairwise spearman correlations of exposures between all signatures detected in the POG570 cohort (n=482 samples). Sample sizes are limited for each correlation (tile) by the number of samples that have evidence of both signatures, as displayed in Fig. 4a.
Extended Data Fig. 7 Prior therapy shapes the tumor genomic landscape.
a, DNA repair genes most frequently mutated across the POG570 cohort (more than 1% of samples), and the number of mutated DNA repair genes per sample. b, Genomic instability (HRD) in patients with mutations in all DNA repair pathways. c, Genomic instability and therapy duration in tumors with prior genotoxic treatment (Wilcoxon rank sum test). d, Exposure of signature SBS31 in samples pre-treated with cis- or carboplatin, separated by tumor type. P values are calculated by two-sided Wilcoxon rank sum tests, comparing the treated and non-treated samples, with no correction. e-f, Prior treatment with platinum (for 2 months-1 year) and HRD status in samples with DBS5 (e) and ID6 (f) signature exposure. Samples were defined as HR deficient if they had a somatic or germline variant in an HR gene (Supplementary Table 7) and exhibited an HRD score > 35, corresponding to the 70th percentile of this cohort. g, Signature SBS2 exposure in BRCA tumors by ER (estrogen receptor) status. h, APOBEC3a expression (TPM) and prior tamoxifen therapy duration in BRCA tumors. DNA repair genes and pathways are defined in Supplementary Table 6: SSA, Single strand annealing: NHEJ, Non-homologous end joining; HDR, Homology directed repair; MMEJ, microhomology mediated end joining; NER, nucleotide excision repair; CCR, cell cycle regulation; DSB, DSB chromatin signaling; ISCR, Interstrand crosslink repair; TS, translesion synthesis; SSBR, single strand break repair; MMR, Mismatch repair; BER, Base excision repair. Boxplots in c, e-h represent the median, upper and lower quartiles of the distribution, and whiskers represent the limits of the distribution (1.5 * interquartile range). All P values presented on boxplots are determined by a two-sided Wilcoxon rank sum test, unless otherwise specified.
Extended Data Fig. 8 Germline events.
a, Type and frequency of germline variants detected in the POG570 cohort (variants found in the 27 genes with germline events shown in Fig. 5a. b, Expression of genes in patients with germline events, compared to the POG570 cohort. A percentile less than 25th (0.25) indicates low expression. c, Exposure of signatures SBS30 and SBS18 in patients with germline, and germline and somatic variants, compared with the POG570 cohort.
Extended Data Fig. 9 Tumor microenvironment.
a, Relationship between tumor content and total predicted immune cell content. b, Cox proportional hazards model of overall survival from advanced disease diagnosis for all patients with CIBERSORT scores (n=568), taking tumor type, immune cluster, and tumor content into consideration. n values for individual groups are shown by the N column. Horizontal lines indicate the 95% confidence interval. c, Shared TCRβ sequences. Samples are displayed around the perimeter, where width represents the frequency of the shared clone in each sample, and joining lines indicate clones shared between samples. The two samples sharing the dominant CSARESTSDPKNEQFF clonotype are identifiable as the two dominant light blue arcs. d, Summary of tumor types and immune checkpoint inhibitors patients received after their POG biopsy. Drug targets and combinations are listed in Methods. e, Probability of continued therapy for the immunotherapy-treated cohort based on exonic mutation burden (n=76) and combined T cell scores (n=57) (see Methods). The p value for e was determined using a log-rank test, and n for each group is displayed in the table under the curves. Tumor types and biopsy sites as described in Fig. 1. All statistical tests are two-sided.
Supplementary information
Supplementary Information
Supplementary Methods.
Supplementary Tables
Supplementary Tables 1–9.
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Source Data Extended Data Fig. 9
Statistical Source Data
Rights and permissions
About this article
Cite this article
Pleasance, E., Titmuss, E., Williamson, L. et al. Pan-cancer analysis of advanced patient tumors reveals interactions between therapy and genomic landscapes. Nat Cancer 1, 452–468 (2020). https://doi.org/10.1038/s43018-020-0050-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-020-0050-6
This article is cited by
-
Integrative single-cell transcriptomic analyses reveal the cellular ontological and functional heterogeneities of primary and metastatic liver tumors
Journal of Translational Medicine (2024)
-
CDK4/6i-treated HR+/HER2- breast cancer tumors show higher ESR1 mutation prevalence and more altered genomic landscape
npj Breast Cancer (2024)
-
Cell cycle gene alterations associate with a redistribution of mutation risk across chromosomal domains in human cancers
Nature Cancer (2024)
-
Liver tropism of ER mutant breast cancer is characterized by unique molecular changes and immune infiltration
Breast Cancer Research and Treatment (2024)
-
A platform-independent AI tumor lineage and site (ATLAS) classifier
Communications Biology (2024)