Abstract
Genetic-driven deregulation of the Wnt pathway is crucial but not sufficient for colorectal cancer (CRC) tumorigenesis. Here, we show that environmental glutamine restriction further augments Wnt signaling in APC-mutant intestinal organoids to promote stemness, and leads to adenocarcinoma formation in vivo via decreasing intracellular α-ketoglutarate (αKG) levels. αKG supplementation is sufficient to rescue low-glutamine-induced stemness and Wnt hyperactivation. Mechanistically, we found that αKG promotes hypomethylation of DNA and histone H3K4me3, leading to an upregulation of differentiation-associated genes and downregulation of Wnt target genes, respectively. Using organoids derived from patients with CRC and several in vivo CRC tumor models, we show that αKG supplementation suppresses Wnt signaling and promotes cellular differentiation, thereby significantly restricting tumor growth and extending survival. Together, our results reveal how the metabolic microenvironment impacts Wnt signaling and identify αKG as a potent antineoplastic metabolite for potential differentiation therapy for patients with CRC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
DNA sequencing and RNA-Seq data that support the findings of this study have been deposited in the Gene Expression Omnibus under accession code GSE140270. SuperSeries GSE140270 is composed of the following SubSeries: GSE140263, GSE140264, GSE140265, GSE140266, GSE140267 and GSE140269. Metabolomics raw data are included in Supplementary Table 5. Source data for Figs. 1–3 and 5–7 and Extended Data Figs. 1–7 are provided with the paper. All other data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Fearon, E. R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. 6, 479–507 (2011).
Schepers, A. & Clevers, H. Wnt signaling, stem cells, and cancer of the gastrointestinal tract. Cold Spring Harb. Perspect. Biol. 4, a007989 (2012).
Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521, 43–47 (2015).
Kinzler, K. W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
De Sousa e Melo, F. et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680 (2017).
Medema, J. P. Targeting the colorectal cancer stem cell. N. Engl. J. Med. 377, 888–890 (2017).
Vermeulen, L. et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12, 468–476 (2010).
Pate, K. T. et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 33, 1454–1473 (2014).
Gao, P. et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765 (2009).
Huang, F., Zhang, Q., Ma, H., Lv, Q. & Zhang, T. Expression of glutaminase is upregulated in colorectal cancer and of clinical significance. Int. J. Clin. Exp. Pathol. 7, 1093–1100 (2014).
Kim, M. H. & Kim, H. The roles of glutamine in the intestine and its implication in intestinal diseases. Int. J. Mol. Sci. 18, E1051 (2017).
Le, A. et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 15, 110–121 (2012).
Carey, B. W., Finley, L. W., Cross, J. R., Allis, C. D. & Thompson, C. B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416 (2015).
Pan, M. et al. Regional glutamine deficiency in tumours promotes dedifferentiation through inhibition of histone demethylation. Nat. Cell Biol. 18, 1090–1101 (2016).
Tran, T. Q., Lowman, X. H. & Kong, M. Molecular pathways: metabolic control of histone methylation and gene expression in cancer. Clin. Cancer Res. 23, 4004–4009 (2017).
Altman, B. J., Stine, Z. E. & Dang, C. V. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 (2016).
Denkert, C. et al. Metabolite profiling of human colon carcinoma—deregulation of TCA cycle and amino acid turnover. Mol. Cancer 7, 72 (2008).
Schuijers, J. et al. Ascl2 acts as an R-Spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell 16, 158–170 (2015).
Gregorieff, A. & Clevers, H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 19, 877–890 (2005).
Voronov, E. & Apte, R. N. IL-1 in colon inflammation, colon carcinogenesis and invasiveness of colon cancer. Cancer Microenviron. 8, 187–200 (2015).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Tse, J. W. T., Jenkins, L. J., Chionh, F. & Mariadason, J. M. Aberrant DNA methylation in colorectal cancer: what should we target? Trends Cancer 3, 698–712 (2017).
Kim, R., Sheaffer, K. L., Choi, I., Won, K. J. & Kaestner, K. H. Epigenetic regulation of intestinal stem cells by Tet1-mediated DNA hydroxymethylation. Genes Dev. 30, 2433–2442 (2016).
Ashktorab, H. & Brim, H. DNA methylation and colorectal cancer. Curr. Colorectal Cancer Rep. 10, 425–430 (2014).
Morris, L. G. et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat. Genet. 45, 253–261 (2013).
Baehs, S. et al. Dickkopf-4 is frequently down-regulated and inhibits growth of colorectal cancer cells. Cancer Lett. 276, 152–159 (2009).
TeSlaa, T. et al. α-Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab. 24, 485–493 (2016).
Salz, T. et al. hSETD1A regulates Wnt target genes and controls tumor growth of colorectal cancer cells. Cancer Res. 74, 775–786 (2014).
Sierra, J., Yoshida, T., Joazeiro, C. A. & Jones, K. A. The APC tumor suppressor counteracts β-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 20, 586–600 (2006).
Ring, A., Kim, Y. M. & Kahn, M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev. 10, 512–525 (2014).
Degos, L. & Wang, Z. Y. All trans retinoic acid in acute promyelocytic leukemia. Oncogene 20, 7140–7145 (2001).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Dow, L. E. et al. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell 161, 1539–1552 (2015).
De Rosa, M. et al. Genetics, diagnosis and management of colorectal cancer (Review). Oncol. Rep. 34, 1087–1096 (2015).
Moser, A. R. et al. ApcMin: a mouse model for intestinal and mammary tumorigenesis. Eur. J. Cancer 31A, 1061–1064 (1995).
Kamphorst, J. J. et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75, 544–553 (2015).
Roberts, E. et al. Amino acids in epidermal carcinogenesis in mice. Cancer Res. 9, 350–353 (1949).
Miyo, M. et al. Metabolic adaptation to nutritional stress in human colorectal cancer. Sci. Rep. 6, 38415 (2016).
Reid, M. A. et al. The B55α subunit of PP2A drives a p53-dependent metabolic adaptation to glutamine deprivation. Mol. Cell 50, 200–211 (2013).
Tran, T. Q. et al. Tumor-associated mutant p53 promotes cancer cell survival upon glutamine deprivation through p21 induction. Oncogene 36, 1991–2001 (2017).
Yang, Y. et al. MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat. Commun. 10, 809 (2019).
Lowman, X. H. et al. p53 promotes cancer cell adaptation to glutamine deprivation by upregulating Slc7a3 to increase arginine uptake. Cell Rep. 26, 3051–3060.e4 (2019).
Carnero, A. & Lleonart, M. The hypoxic microenvironment: a determinant of cancer stem cell evolution. BioEssays 38, S65–S74 (2016).
Sullivan, R., Pare, G. C., Frederiksen, L. J., Semenza, G. L. & Graham, C. H. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol. Cancer Ther. 7, 1961–1973 (2008).
Raa, A. et al. Hyperoxia retards growth and induces apoptosis and loss of glands and blood vessels in DMBA-induced rat mammary tumors. BMC Cancer 7, 23 (2007).
Stuhr, L. E. et al. Hyperoxia retards growth and induces apoptosis, changes in vascular density and gene expression in transplanted gliomas in nude rats. J. Neurooncol. 85, 191–202 (2007).
Stępień, K., Ostrowski, R. P. & Matyja, E. Hyperbaric oxygen as an adjunctive therapy in treatment of malignancies, including brain tumours. Med. Oncol. 33, 101 (2016).
Miyoshi, Y. et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum. Mol. Genet. 1, 229–233 (1992).
Voloshanenko, O. et al. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nat. Commun. 4, 2610 (2013).
Schneikert, J., Grohmann, A. & Behrens, J. Truncated APC regulates the transcriptional activity of β-catenin in a cell cycle dependent manner. Hum. Mol. Genet. 16, 199–209 (2007).
Pai, R. et al. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med. 8, 289–293 (2002).
Janssen, K. P. et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 131, 1096–1109 (2006).
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
Seshagiri, S. et al. Recurrent R-Spondin fusions in colon cancer. Nature 488, 660–664 (2012).
The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Krishnamurthy, N. & Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat. Rev. 62, 50–60 (2018).
Jimeno, A. et al. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin. Cancer Res. 23, 7490–7497 (2017).
O’Rourke, K. P., Ackerman, S., Dow, L. E. & Lowe, S. W. Isolation, culture, and maintenance of mouse intestinal stem cells. Bio Protoc. 6, e1733 (2016).
Van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Tran, T. Q. et al. Glutamine deficiency induces DNA alkylation damage and sensitizes cancer cells to alkylating agents through inhibition of ALKBH enzymes. PLoS Biol. 15, e2002810 (2017).
Liu, X., Ser, Z. & Locasale, J. W. Development and quantitative evaluation of a high-resolution metabolomics technology. Anal. Chem. 86, 2175–2184 (2014).
Tong, K. et al. Degree of tissue differentiation dictates susceptibility to BRAF-driven colorectal cancer. Cell Rep. 21, 3833–3845 (2017).
Acknowledgements
We thank members of the Kong laboratory and the Waterman laboratory, as well as E. J. Stanbridge, for helpful comments on the manuscript. We thank D. T. Florina for assistance with the animal experiments, and the L. Dow Laboratory (Weill Cornell) for the AKP organoids. This research was supported by funds from the NIH (R01CA183989 and R01GM132142 to M.K.; R01CA17765 to M.L.W. and R.A.E.; and U54CA217378 to M.L.W.) and American Cancer Society (grant number RSG-16-085-01-TBE to M.K.). E.A.H. is supported by the American Cancer Society (PFDDC-132846). A.N.H. is supported by NSF grant DGE‐1321846 and NIH grant T32CA009054. Research reported here includes work carried out in Core Facilities at the University of California Irvine, supported by the NIH under grant number P30CA062203.
Author information
Authors and Affiliations
Contributions
T.Q.T. conceived of the project, designed and performed the majority of the experiments and wrote the manuscript. M.K. conceived of the project, supervised the study, designed the experiments and wrote the manuscript. E.A.H. performed the ChIP experiments, metabolite measurements and animal studies and contributed to manuscript preparation. A.N.H. provided materials, performed the PDO culture experiments and contributed to manuscript preparation. H.L. performed the bioinformatic analyses. M.B.I.G. and Y.Y. assisted with the animal studies. X.H.L. and A.M.O. assisted with qPCR and sample preparation. S.Y.L. provided clinical samples for the PDOs. R.A.E. assisted with the pathological analysis. M.L.W. provided conceptual advice on the experimental design and assisted with manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
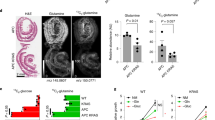
Extended Data Fig. 1 The effect of glutamine starvation on ApcMin/+ and wildtype organoids.
a, Relative glutamine uptake in ApcMin/+ organoids and wildtype organoids (n=3 biologically independent samples) and relative glutamine uptake in AKP organoids upon doxycycline addition (n=4 biologically independent samples). b, Percentage of cystic organoid in ApcMin/+ organoids upon glutamine deprivation (0.2 mM and 0.4 mM) overtime (n=7 biologically independent cultures). c, Percentage of cystic organoids and organoid number of ApcMin/+ organoids treated with CB-839 for 1 week (n=3 biologically independent cultures). d, Percentage of wildtype organoids with cystic morphology after 4 passages in low glutamine conditon (n=6 biologically independent cultures). e, qPCR analysis of Axin2 in wildtype organoids cultured in control or low glutamine medium for 1 week. Data from n=3 independent experiments with a line marking the mean value. (f,g) Control and glutamine-starved wildtype organoids were dissociated into single cells, and equal number of organoid-derived cells were cultured in organoid medium with 3 mM or 0.3 mM glutamine (low gln). Secondary organoid formation and percentage of cystic organoids are shown (n=9 biologically independent cultures). Data in a-e,g represent means ± SD, p values were determined by two-tailed unpaired Student’s t-test. Scale bars, 1000 μm (c,d,f). Source data are provided for a-e,g.
Extended Data Fig. 2 Genetic alterations do not contribute to low-glutamine induced stemness.
a, Identified genetic alterations in glutamine-starved ApcMin/+ organoids compared to paired control organoids as determined by exome sequencing. b, Brightfield images of control and glutamine-starved ApcMin/+organoids after 12 passages. Results are representative of three biologically independent cultures. c, Immunoblotting for full length and truncated Apc protein in control and glutamine starved ApcMin/+ organoids after 8 passages. Results are representative of three independent experiments. d, Representative images and percentage of cells with full-length Apc protein based on immunofluorescent staining with C-terminus Apc antibody in wildtype organoids, tumour organoids derived from adenomas derived in ApcMin/+ mice, and ApcMin/+organoids from healthy tissues in control medium and upon glutamine deprivation (n=4 biologically independent cultures), data represent means ± SD. (e,f) Representative images and percentage of shApc /KrasG12D/p53fl/fl (AKP) organoid with crypts cultured in control or low glutamine medium for 10 days (n=5 biologically independent cultures). g, qPCR analysis of Krt20 and Lgr5 in a similar experiment described in e after 3 days of glutamine deprivation. Data from n=2 independent experiments with a line marking the mean value. h, Hierarchical clustering of significant differentiated gene expression of ApcMin/+ organoids cultured in control or low-glutamine medium (n=3 biologically independent samples). i, qPCR analysis of the indicated genes in SW620 colon cancer cells (n=3 technical replicates and data represent means) cultured in medium with the indicated glutamine concentration for 3 days. A single experiment is shown that is representative of two independent experiments with similar results. Scale bars, 200 µm (b), 400 μm (d,e). Unprocessed blot images for c and source data for d,f,g,i are provided.
Extended Data Fig. 3 The role of aKG in low-glutamine induced stemness.
a, Schematic diagram of glutamine metabolism. b, Relative metabolite levels as measured by LC-MS in ApcMin/+ organoids cultured in control and low glutamine medium (n=4 biologically independent samples). c, Relative aKG levels in intestinal tumours from ApcMin/+ mice and normal intestinal tissues of wildtype mice (n=5 mice per group). Data in b,c represent means ± SD, and p values were determined by two-tailed unpaired Student’s t-test. (d,e) Relative intracellular aKG and succinate levels in ApcMin/+ organoids upon DM-aKG (n=5 biologically independent samples) or DM-succinate supplementation (n=6 biologically independent samples). f, Immunofluorescent staining for ROS in ApcMin/+ organoids under low glutamine or low glutamine medium supplemented with NAC. Results are representative from three biologically independent samples. g, Control organoids, glutamine-starved organoids treated with or without 3.5 mM DM-aKG were dissociated into single cells. An equal number of organoid-derived cells were cultured, and secondary organoid formation (n=6 biologically independent cultures) and cell proliferation (n=3 biologically independent cultures) were measured after 1 week and are shown. p values were determined by two-tailed unpaired Student’s t-test. h, Immunoblotting for Lgr5 and (i) qPCR analysis for Axin2 expression in ApcMin/+ organoid cultured in control and low glutamine medium with or without DM-aKG (n= 2 independent experiments with a line marking the mean value). Box plots in d,e,g show the maximum, third quartile, median, first quartile and minimum values, and the p values were determined by two-tailed unpaired Student’s t-test. Scale bar, 400 μm f, 1000 μm g. Unprocessed blot images for h and source data for b-e and g,i are provided.
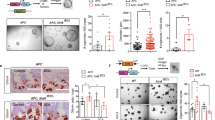
Extended Data Fig. 4 The effect of aKG and glutamine supplementation on intestinal differentiation.
a, Representative brightfield images and immunofluorescent staining of the differentiation marker Krt20 in ApcMin/+ organoids treated with 3 mM DM-aKG for 3 days. Results are representative of three independent experiments. b, Representative images and relative organoid number of control ApcMin/+ organoid or glutamine-starved ApcMin/+ organoids upon 2 mM DM-aKG treatment or 6mM glutamine addition for 1 week (n=3 biologically independent cultures). Data represent means ± SD, and the p values were determined by two-tailed unpaired Student’s t-test. c, Overlapping gene expression profile of ApcMin/+ organoids cultured in low glutamine medium or treated with aKG reveals opposing regulation on Wnt target genes and intestinal differentiation related genes. Scale bars, 1000 μm (Brightfield), 200 μm (Immunofluorescence). Source data are provided for b.
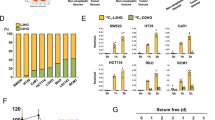
Extended Data Fig. 5 aKG promotes hypomethylation of histone and DNA in CRC cells.
a, qPCR analysis of Axin2 in control and glutamine-starved organoids treated with 1 μM decitabine for 3 days. Data from n=2 independent experiments with a line marking the mean value. (b,c) Dot blot analysis of 5meC levels in ApcMin/+ organoids in control and low glutamine medium and SW620 cells in control, low glutamine medium or low glutamine medium supplemented with 8mM DM-aKG. Results are representative from two independent experiments. d, Heatmap of the differential methylated regions (different methylated ratio >±20%) in SW620 cells upon 8 mM DM-aKG treatment for 3 days. Beta value of the methylation ratio are shown (n=2 biologically independent samples). e, qPCR analysis of Dkk4 in ApcMin/+ organoids treated with 3.5 mM DM-aKG or 1 μM decitabine (n=3 technical replicates). A repeat experiment showed similar results. f, Dot blot analysis of 5meC levels in SW620 cells treated with DM-aKG (left), MeDIP experiment with 5meC antibody for DKK4 promoter in SW620 cells upon 8 mM DM-aKG treatment (right). Data show means ± SD of n=4 technical replicates. Results are representative of two independent experiments. g, qPCR analysis of TET1 expression in SW620 cells transfected with control siRNA or TET1 siRNA (data show means of n=3 technical replicates). h, qPCR analysis of DKK4 and LGR5 expression in control SW620 cells or TET1 siRNA knockdown cells following DM-aKG treatment. Data from n=2 independent experiments with a line marking the mean value. i, Representative immunoblot of histone methylation in SW620 cells treated with DM-aKG from two independent experiments. j, ChIP analysis of H3K4 levels on promoter regions of AXIN2 and MYC in SW620 cells in response to 8mM DM-aKG treatment for 3 days (n=4 technical replicates). k, Representative immunoblot of H3K4me3 in SW620 cells in control, low-glutamine medium or low glutamine medium supplemented with 8 mM DM-aKG from two independent experiments. l, ChIP analysis of H3K4me3 levels on promoter regions of AXIN2 and MYC in SW620 cells in response to glutamine starvation after 1 week (n=4 technical replicates). Results in j and l represent means ± SD and are representative of two independent experiments. Unprocessed blot images are provided for b,c,f, i and k. Source data are provided for a,e-h,j, and l.
Extended Data Fig. 6 DM-aKG treatment inhibits initiation and growth of PDOs.
a, Clinical information on PDOs used in the study. b, Immunoblot probed for Apc protein in different PDOs. c, Relative organoid size (n=50 organoids) and d, representative images of four biologically independent cultures of T23 PDO treated with 6 mM DM-aKG for 7 days, followed by metabolite wash-out and subsequent culture for 7 days. Data in c represent means ± SD. Scale bar, 400 μm (d). Unprocessed blot images for b and source data for c is provided.
Extended Data Fig. 7 The effect of DM-aKG treatment in mice.
a, Body weight and histological analysis of wildtype mice treated with 400 mg/kg DM-aKG via IP injection for more than 2 months (n=4 mice per group). b, Representative IHC staining for Cyclin D1 in intestinal tissues collected from ApcMin/+ mice treated with DM-aKG from three mice per group. c, Gene expression analysis from RNA sequencing performed on the intestinal tissues of wildtype mice (n=7 mice), ApcMin/+ mice (n=7 mice), and ApcMin/+ mice treated with DM-aKG (n=6 mice). d, Body weight changes and images of liver and spleen from ApcMin/+ mice treated with DM-aKG (n=5 mice per group). e, Liver and kidney function of wildtype mice treated with 15 mg/ml DM-aKG supplemented in drinking water for more than 4 months (n=5 mice per group). Data shown in a,d,e are means ± SD. The p values in e were determined by two-tailed unpaired Student’s t-test. Source data are provided for a,d,e.
Supplementary information
Supplementary Tables
Supplementary Tables 1–5.
Source data
Source Data Fig. 1
Statistical source data
Source Data Fig. 1
Unprocessed western blots and/or gels
Source Data Fig. 2
Source data
Source Data Fig. 3
Source data
Source Data Fig. 5
Source data
Source Data Fig. 6
Source data
Source Data Fig. 6
Unprocessed western blots and/or gels
Source Data Fig. 7
Source data
Source Data Extended Data Fig. 1
Source data
Source Data Extended Data Fig. 2
Source data
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels
Source Data Extended Data Fig. 3
Source data
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels
Source Data Extended Data Fig. 4
Source data
Source Data Extended Data Fig. 5
Source data
Source Data Extended Data Fig. 5
Unprocessed western blots and/or gels
Source Data Extended Data Fig. 6
Source data
Source Data Extended Data Fig. 6
Unprocessed western blots and/or gels
Source Data Extended Data Fig. 7
Source data
Rights and permissions
About this article
Cite this article
Tran, T.Q., Hanse, E.A., Habowski, A.N. et al. α-Ketoglutarate attenuates Wnt signaling and drives differentiation in colorectal cancer. Nat Cancer 1, 345–358 (2020). https://doi.org/10.1038/s43018-020-0035-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-020-0035-5
This article is cited by
-
Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer
Molecular Cancer (2023)
-
Ketoglutaric acid can reprogram the immunophenotype of triple-negative breast cancer after radiotherapy and improve the therapeutic effect of anti-PD-L1
Journal of Translational Medicine (2023)
-
Metabolic determinants of tumour initiation
Nature Reviews Endocrinology (2023)
-
The Fe–S cluster assembly protein IscU2 increases α-ketoglutarate catabolism and DNA 5mC to promote tumor growth
Cell Discovery (2023)
-
IDH2, a novel target of OGT, facilitates glucose uptake and cellular bioenergy production via NF-κB signaling to promote colorectal cancer progression
Cellular Oncology (2023)