Abstract
The success of molecular therapies targeting specific metabolic pathways in cancer is often limited by the plasticity and adaptability of metabolic networks. Here we show that pharmacologically induced lipotoxicity represents a promising therapeutic strategy for the treatment of hepatocellular carcinoma (HCC). LXRα-induced liponeogenesis and Raf-1 inhibition are synthetic lethal in HCC owing to a toxic accumulation of saturated fatty acids. Raf-1 was found to bind and activate SCD1, and conformation-changing DFG-out Raf inhibitors could disrupt this interaction, thereby blocking fatty acid desaturation and inducing lethal lipotoxicity. Studies in genetically engineered and nonalcoholic steatohepatitis-induced HCC mouse models and xenograft models of human HCC revealed that therapies comprising LXR agonists and Raf inhibitors were well tolerated and capable of overcoming therapy resistance in HCC. Conceptually, our study suggests pharmacologically induced lipotoxicity as a new mode for metabolic targeting of liver cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. For the e:Med-HCC-1 clinical study (NCT02372162), all data can be requested for research purposes from Michael Bitzer (michael.bitzer@uni-tuebingen.de). Deep sequencing (RNA-seq) data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) under accession codes GSE162322 and GSE162324. For the molecular modeling studies, all data are deposited in the Zenodo repository (https://doi.org/10.5281/zenodo.3253878, under accession code 3253878) and can also be requested for research purposes from Antti Poso (antti.poso@uef.fi). Associated raw data for Figs. 1b,c and 2b and Extended Data Fig. 3d are provided as supplementary tables. The gating strategy for flow cytometry is depicted in Extended Data Figs. 2a,b and 6f–h. Source data are provided with this paper.
References
Schulze, A. & Harris, A. L. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 491, 364–373 (2012).
Luengo, A., Gui, D. Y. & Vander Heiden, M. G. Targeting metabolism for cancer therapy. Cell Chem. Biol. 24, 1161–1180 (2017).
Vriens, K. et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 566, 403–406 (2019).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2, 16018 (2016).
Rosmorduc, O. & Fartoux, L. HCC and NASH: how strong is the clinical demonstration? Clin. Res. Hepatol. Gastroenterol. 36, 202–208 (2012).
Anstee, Q. M., Reeves, H. L., Kotsiliti, E., Govaere, O. & Heikenwalder, M. From NASH to HCC: current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 16, 411–428 (2019).
Yau, T., Chan, P., Epstein, R. & Poon, R. T. Evolution of systemic therapy of advanced hepatocellular carcinoma. World J. Gastroenterol. 14, 6437–6441 (2008).
Llovet, J. M., Montal, R., Sia, D. & Finn, R. S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 15, 599–616 (2018).
Raoul, J. L. et al. Systemic therapy for intermediate and advanced hepatocellular carcinoma: sorafenib and beyond. Cancer Treat. Rev. 68, 16–24 (2018).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Finn, R. S. et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 38, 193–202 (2020).
Rudalska, R. et al. In vivo RNAi screening identifies a mechanism of sorafenib resistance in liver cancer. Nat. Med. 20, 1138–1146 (2014).
Dauch, D. et al. A MYC−Aurora kinase A protein complex represents an actionable drug target in p53-altered liver cancer. Nat. Med. 22, 744–753 (2016).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Seehawer, M. et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 562, 69–75 (2018).
Peet, D. J., Janowski, B. A. & Mangelsdorf, D. J. The LXRs: a new class of oxysterol receptors. Curr. Opin. Genet. Dev. 8, 571–575 (1998).
Hong, C. & Tontonoz, P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat. Rev. Drug Discov. 13, 433–444 (2014).
Schultz, J. R. et al. Role of LXRs in control of lipogenesis. Genes Dev. 14, 2831–2838 (2000).
Peet, D. J. et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell 93, 693–704 (1998).
Pencheva, N., Buss, C. G., Posada, J., Merghoub, T. & Tavazoie, S. F. Broad-spectrum therapeutic suppression of metastatic melanoma through nuclear hormone receptor activation. Cell 156, 986–1001 (2014).
Janowski, B. A. et al. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc. Natl Acad. Sci. USA 96, 266–271 (1999).
Liu, L. et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 66, 11851–11858 (2006).
Wilhelm, S. M. et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64, 7099–7109 (2004).
Griffett, K. et al. The LXR inverse agonist SR9238 suppresses fibrosis in a model of non-alcoholic steatohepatitis. Mol. Metab. 4, 353–357 (2015).
Xue, W. et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 514, 380–384 (2014).
Terasaka, N. et al. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 536, 6–11 (2003).
Waizenegger, I. C. et al. A novel RAF kinase inhibitor with DFG-out-binding mode: high efficacy in BRAF-mutant tumor xenograft models in the absence of normal tissue hyperproliferation. Mol. Cancer Ther. 15, 354 (2016).
Faivre, S., Demetri, G., Sargent, W. & Raymond, E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 6, 734–745 (2007).
Villa, G. R. et al. An LXR–cholesterol axis creates a metabolic co-dependency for brain cancers. Cancer Cell 30, 683–693 (2016).
Chen, K. F. et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 337, 155–161 (2011).
Porstmann, T. et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236 (2008).
Kay, H. Y. et al. Nrf2 inhibits LXRα-dependent hepatic lipogenesis by competing with FXR for acetylase binding. Antioxid. Redox Signal. 15, 2135–2146 (2011).
Holohan, C., Van, S. S., Longley, D. B. & Johnston, P. G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013).
Bovenga, F., Sabba, C. & Moschetta, A. Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metab. 21, 517–526 (2015).
Harriman, G. et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc. Natl Acad. Sci. USA 113, E1796–E1805 (2016).
Pommier, A. J. et al. Liver X receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene 29, 2712–2723 (2010).
Nguyen, T. T. T. et al. Activation of LXR receptors and inhibition of TRAP1 causes synthetic lethality in solid tumors. Cancers https://doi.org/10.3390/cancers11060788 (2019).
Wang, D., Wei, Y. & Pagliassotti, M. J. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 147, 943–951 (2006).
Ricchi, M. et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroenterol. Hepatol. 24, 830–840 (2009).
Zambo, V. et al. Lipotoxicity in the liver. World J. Hepatol. 5, 550–557 (2013).
Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 (2012).
McCullough, K. D., Martindale, J. L., Klotz, L. O., Aw, T. Y. & Holbrook, N. J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 21, 1249–1259 (2001).
Holze, C. et al. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 19, 130–140 (2018).
Souers, A. J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208 (2013).
Ntambi, J. M. & Miyazaki, M. Recent insights into stearoyl-CoA desaturase-1. Curr. Opin. Lipidol. 14, 255–261 (2003).
Zhang, X. et al. Liver X receptor activation increases hepatic fatty acid desaturation by the induction of SCD1 expression through an LXRα–SREBP1c-dependent mechanism. J. Diabetes 6, 212–220 (2014).
Mziaut, H., Korza, G. & Ozols, J. The N terminus of microsomal Δ9 stearoyl-CoA desaturase contains the sequence determinant for its rapid degradation. Proc. Natl Acad. Sci. USA 97, 8883–8888 (2000).
Kato, H., Sakaki, K. & Mihara, K. Ubiquitin–proteasome-dependent degradation of mammalian ER stearoyl-CoA desaturase. J. Cell Sci. 119, 2342–2353 (2006).
Peng, S. B. et al. Inhibition of RAF isoforms and active dimers by LY3009120 leads to anti-tumor activities in RAS or BRAF mutant cancers. Cancer Cell 28, 384–398 (2015).
Shao, W. et al. Antitumor properties of RAF709, a highly selective and potent inhibitor of RAF kinase dimers, in tumors driven by mutant RAS or BRAF. Cancer Res. 78, 1537–1548 (2018).
Gibney, G. T. & Zager, J. S. Clinical development of dabrafenib in BRAF mutant melanoma and other malignancies. Expert Opin. Drug Metab. Toxicol. 9, 893–899 (2013).
King, A. J. et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 66, 11100–11105 (2006).
Yeh, T. C. et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin. Cancer Res. 13, 1576–1583 (2007).
Agianian, B. & Gavathiotis, E. Current insights of BRAF inhibitors in cancer. J. Med. Chem. 61, 5775–5793 (2018).
Xin, Z. et al. Discovery of piperidine-aryl urea-based stearoyl-CoA desaturase 1 inhibitors. Bioorg. Med. Chem. Lett. 18, 4298–4302 (2008).
Sebolt-Leopold, J. S. & Herrera, R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer 4, 937–947 (2004).
Wolf, M. J. et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 26, 549–564 (2014).
Nwosu, Z. C. et al. Liver cancer cell lines distinctly mimic the metabolic gene expression pattern of the corresponding human tumours. J. Exp. Clin. Cancer Res. 37, 211 (2018).
Preziosi, M. E. et al. In vivo screen identifies LXR agonism potentiates sorafenib killing of hepatocellular carcinoma. Preprint at bioRxiv https://doi.org/10.1101/668350 (2019).
Kirchgessner, T. G. et al. Pharmacological characterization of a novel liver X receptor agonist with partial LXRα activity and a favorable window in nonhuman primates. J. Pharmacol. Exp. Ther. 352, 305–314 (2015).
Carlson, C. M., Frandsen, J. L., Kirchhof, N., McIvor, R. S. & Largaespada, D. A. Somatic integration of an oncogene-harboring Sleeping Beauty transposon models liver tumor development in the mouse. Proc. Natl Acad. Sci. USA 102, 17059–17064 (2005).
Zender, L. et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125, 1253–1267 (2006).
Watnick, R. S., Cheng, Y. N., Rangarajan, A., Ince, T. A. & Weinberg, R. A. Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell 3, 219–231 (2003).
Pelossof, R. et al. Prediction of potent shRNAs with a sequential classification algorithm. Nat. Biotechnol. 35, 350–353 (2017).
Fellmann, C. et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep. 5, 1704–1713 (2013).
Naito, Y., Hino, K., Bono, H. & Ui-Tei, K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31, 1120–1123 (2015).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Feoktistova, M., Geserick, P. & Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, prot087379 (2016).
Mehlem, A., Hagberg, C. E., Muhl, L., Eriksson, U. & Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 8, 1149–1154 (2013).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Babicki, S. et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 44, W147–W153 (2016).
Mendez, J. & Stillman, B. Chromatin association of human origin recognition complex, Cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20, 8602–8612 (2000).
Friesner, R. A. et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein–ligand complexes. J. Med. Chem. 49, 6177–6196 (2006).
Acknowledgements
We thank E. Rist, P. Schiemann, A. Heinrich, E. Enzinger, O. Hihn, W. Albrecht, L. Herrmann, P. Wenk, F. Iglauer, S. Gerold, M. A. Jarboui, U. Koppenhoefer, S. Poths, F. J. Hilke, D. Quetting, M. Riebold and all members of the Zender department for fruitful discussions and/or technical assistance. Furthermore, we thank M. Eilers and J. Zuber for providing vectors, the c.ATG facility of Tuebingen University for sequencing analyses and the CSC-IT Center for Science, Finland, for computational resources. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (FOR2314 (project number 267467939; D.D., L.Z., A.S., N.P., B.P., S.L., M.T.R., A.P., N.P.M.), SFB-TR209-314905040 (D.D., L.Z., N.P.M., M.B.), SFB-TR240 (L.Z., B.P.), Gottfried Wilhelm Leibniz Program (L.Z.)), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s excellence strategy EXC 2180-390900677 (Image Guided and Functionally Instructed Tumor Therapies (iFIT) (L.Z., B.P., D.D., S.L., N.P., M.B., A.P., N.P.M.)) and the German Ministry for Education and Research (BMBF) (eMed/Multiscale HCC (L.Z., B.P., N.P.M., M.B.), Neuroallianz (S.L.)). Further funding was provided by the Landesstiftung Baden-Wuerttemberg (Improve CRC (D.D., S.L., L.Z.)), the European Research Council (CholangioConcept (L.Z.)), the German Center for Translational Cancer Research (DKTK (S.L., L.Z.)) and the Ministry of Science, Research and Arts of Baden-Wuerttemberg (MWK-BW (S.L., M.B.)). Furthermore, S.L. is supported by the Robert Bosch Foundation and the Interfaculty Centre for Pharmacogenomics and Pharma Research (ICEPHA), and B.P. is supported by the Werner Siemens Foundation and the DFG (project number 403462768).
Author information
Authors and Affiliations
Contributions
The study was designed by D.D. and L.Z. The research was conducted by R.R., J.H., M.T.S., S.K., S.Z., L.T., F.H., M.F., W.C., L.d’A., E.E., M.H., W.S., A.D., T.-W.K. and D.D. Histopathological analyses were performed by M.T.R. Human HCC samples were collected by M.B. Molecular modeling was conducted by T.K. and A.P. N.P.M., S.L., B.P., N.P. and A.S. contributed to research design and supervised experiments. D.D. and L.Z. supervised all research. The manuscript was written by D.D. and L.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Cancer thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The treatment response of sorafenib in MycOE;NrasG12V-driven HCCs is dependent on LXRα activation.
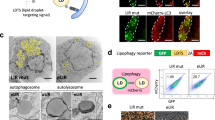
a,b, Treatment of murine, MycOE;NrasG12V-driven HCCs with sorafenib. Representative pictures (a) and H+E staining (b) of MycOE;NrasG12V HCCs in wildtype mice upon a 5-week therapy with sorafenib or the corresponding carrier (n = 3 mice per group). Scale bars, 1 cm, 100 µm. c, Survival of mice with MycOE;NrasG12V HCCs under sorafenib or carrier treatment (Kaplan-Meier curve, statistical significance was calculated using a log-rank test, n = 6 mice per group). d, Overview of LXRß structure with the coactivator peptide from NCOA1 (dark blue) in the AF2 region and sorafenib structure, docked in the ligand-binding pocket (orange). e, Suggested binding mode of sorafenib proposed by molecular modelling into the ligand-binding pocket of LXRß (PDB ID: 1PQ6). Residues contributing towards the interaction are colored by property, hydrophobic side chains are purple and hydrogen bonds are cyan. f, Schematic outline of an LXRα-binding saturation assay. Pulldown of LXRα was performed upon incubation with different concentrations of 3H-sorafenib. This experiment was side-by-side conducted in the presence of 320 µM unlabeled sorafenib to determine unspecific binding. g, Representative western blot analysis of LXRα in individual MycOE;NrasG12V;gN1h3 tumors (cropped blot images, MycOE;NrasG12V;gNC control: n = 3 mice, the western blot was repeated twice with similar results). α-tubulin was used as a loading control. Unprocessed images of the blots and numerical source data are provided as source data files.
Extended Data Fig. 2 Inhibition of Raf improves the therapeutic effect of LXRα activation in liver cancer.
a,b, Gating strategy to quantify LXR activity in HCC cells. FACS analysis of 10 µM sorafenib-treated MycOE;NrasG12V;Cdkn2aARF–/–;p-LXRE-GFP cells. c, LXRE reporter assay in MycOE;NrasG12V;Cdkn2aARF–/–;p-LXRE-GFP cells that were treated for 3 days with different concentrations of sorafenib or T0901317 (GFP measurements in individual cells, values represent mean ± SD, n = 3 independent experiments, nonlinear regression curves, variable slope). d, Reporter assay to compare LXR activation by sorafenib or T0901317 in vivo. Representative photographs of tumor sections stained for native GFP and native RFP (+DAPI) after intrahepatic delivery of pT-CaM-LXRE-G, pT-CaRIN and pSB13 and treatment with carrier, sorafenib or T0901317 (3 d, n = 4 mice per group). Scale bar, 100 µm. e, Establishing Nr1h3 or Nr1h2 gene knockout in MycOE;NrasG12V;Cdkn2aARF–/– HCC cells by transient transfection of cells with pX-458-GFP expressing the corresponding gRNAs and generation of single cell clones. f,g, Representative western blot analysis of LXRα (f) and LXRβ (g) in MycOE;NrasG12V;Cdkn2aARF–/– single cell clones upon transient transfection with pX-458-GFP expressing the corresponding gRNAs (single cell clones, cropped blot images, n = 2 independent experiments). Vinculin and Histone H3 were used as loading controls. h, Treatment of MycOE;NrasG12V;Cdkn2aARF–/–gNr1h3, MycOE;NrasG12V;Cdkn2aARF–/–gNr1h2 or MycOE;NrasG12V;Cdkn2aARF–/–gNC cells for 4 days with T0901317/sorafenib or carrier (quantification of viable cells by crystal violet staining, values represent mean ± SD, n = 3 independent experiments, statistical significance was calculated using two-tailed Student´s t test). i, Treatment of MycOE;NrasG12V;Cdkn2aARF–/– cells for 4 days with sorafenib and/or 24-S hydroxycholesterol, GW3965 or LXR-623 (quantification of viable cells by crystal violet staining, values represent mean ± SD, n = 3 independent experiments, statistical significance was calculated using two-tailed Student´s t test). Unprocessed images of the blots and numerical source data are provided as source data files.
Extended Data Fig. 3 Sorafenib resistant, MycOE;Akt1Myr-driven murine HCCs exert reduced LXRα activity.
a, Co-delivery of a transposon construct encoding for oncogenic Myc and myristoylated Akt1 (Akt1Myr) and pSB13 into C57BL/6 wildtype mice via hydrodynamic tail vein injection. b, Representative western blot analysis of P-Mek1/2 (S217/221) and Mek1/2 in MycOE;Akt1Myr tumors 6 h after treatment with sorafenib or BI-882370 (cropped blot images, n = 2 mice per condition). α-tubulin was used as a loading control. c, Survival of mice with MycOE;Akt1Myr HCCs under sorafenib or carrier treatment (Kaplan-Meier curve, statistical significance was calculated using a log-rank test, n = 6 mice per group). d, mRNA levels of Nr1h3, Abcg1, Abcg5, Abcg8 and Apoe in MycOE;Akt1Myr tumors (compared to MycOE;NrasG12V HCCs, mRNAseq data, n = 3 mice per group, statistical significance was calculated using two-tailed Student´s t test). e, Representative western blot analysis of Akt-1 and LXRα in MycOE;Akt1Myr;Cdkn2aARF–/– and MycOE;NrasG12V;Cdkn2aARF–/– cells (cropped blot images, n = 3 independent experiments). β-actin was used as a loading control. f, LXRE reporter assay in MycOE;Akt1Myr;Cdkn2aARF–/–;p-LXRE-GFP and MycOE;NrasG12V;Cdkn2aARF–/–;p-LXRE-GFP cells (GFP measurements in individual cells, values represent mean ± SD, n = 5 independent experiments, statistical significance was calculated using two-tailed Student´s t test). g, mRNA levels of Nfe2l2 in MycOE;Akt1Myr tumors (compared to MycOE;NrasG12V HCCs, mRNAseq data, n = 3 mice per group, statistical significance was calculated using two-tailed Student´s t test). h, Representative western blot analysis of Nrf2 in MycOE;Akt1Myr;Cdkn2aARF–/– and MycOE;NrasG12V;Cdkn2aARF–/– cells (cropped blot images, n = 3 independent experiments). α-tubulin was used as a loading control. i, Representative western blot analysis of Nrf2 in MycOE;Akt1Myr;Cdkn2aARF–/–shNfe2l2 or MycOE;Akt1Myr;Cdkn2aARF–/–shNC cells upon 4 days of treatment with doxycycline (cropped blot images, n = 3 independent experiments). α-tubulin was used as a loading control. j, LXRE reporter assay in MycOE;Akt1Myr;Cdkn2aARF–/–;p-LXRE-GFP cells 4 days after transient transfection with pT-CaR-shNfe2l2.2406, pT-CaR-shNfe2l2.2272, or pT-CaR-shNC (GFP measurements in RFP positive, individual cells, values represent mean ± SD, n = 5 independent experiments, statistical significance was calculated using two-tailed Student´s t test). k, LXRE reporter assay in MycOE;Akt1Myr;Cdkn2aARF–/–;p-LXRE-GFP cells upon 3 days of treatment with sorafenib or T0901317 (GFP measurements in individual cells, values represent mean ± SD, n = 3 independent experiments, statistical significance was calculated using two-tailed Student´s t test). Unprocessed images of the blots and numerical source data are provided as source data files.
Extended Data Fig. 4 A combination of LXR activation and Raf inhibition blocks MycOE;Akt-1Myr-driven HCC development.
a, Quantification of MycOE;Akt1Myr tumor nodules from mice that were treated for 7 weeks with sorafenib, BI-882370 or carrier +/− T0901317 (values represent mean ± SD, n = 4 mice per group, statistical significance was calculated using two-tailed Student´s t test). b-d, Treatment of MycOE;Akt1Myr;gNr1h3 or MycOE;Akt1Myr;gNC HCCs with T0901317/sorafenib or carrier. Representative pictures of livers upon a 6-week therapy (c, n = 3 mice per group) and survival of mice under treatment (d, Kaplan-Meier curve, statistical significance was calculated using a log-rank test, n = 6 mice per group). Scale bars, 1 cm. Numerical source data are provided as source data files.
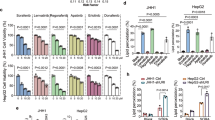
Extended Data Fig. 5 A T0901317/sorafenib combinatorial therapy influences lipid metabolism in liver cancer.
a, Representative pictures of murine Yap1;NrasG12V;Trp53-/- tumors that were treated for 2 weeks with T0901317/sorafenib, carrier, T0901317 or sorafenib and stained with H+E (n = 3 mice per group). Scale bars, 100 µm. b, Representative pictures of MycOE;NrasG12V tumors that were treated for 3 weeks with T0901317/sorafenib, carrier, T0901317 or sorafenib and stained with Oil red O (n = 3 mice per group). Scale bars, 50 µm. c, Representative western blot analysis of Srebp1c and Acetyl-CoA Carboxylase1 (ACACA) in MycOE;NrasG12V;Cdkn2aARF–/– cells upon 3 days of treatment with sorafenib, T0901317 or a combination thereof (cropped blot images, n = 2 independent experiments). α-tubulin was used as a loading control. d, Treatment of MycOE;NrasG12V;Cdkn2aARF–/– cells with the ACACA inhibitor ND-630 (quantification of viable cells by crystal violet staining, values represent mean ± SD, n = 3 independent experiments, statistical significance was calculated using two-tailed Student´s t test). e, Treatment of MycOE;NrasG12V;Cdkn2aARF–/– cells with T0901317/sorafenib +/− cholesterol/methyl-β-cyclodextrin (cholesterol/mbc) (quantification of viable cells by crystal violet staining, values represent mean, n = 6 cell independent experiments, statistical significance was calculated using two-tailed Student´s t test). f, Schematic overview describing the role of Raf in fatty acid metabolism in liver cancer. Unprocessed images of the blots and numerical source data are provided as source data files.
Extended Data Fig. 6 A T0901317/sorafenib therapy results in lipid peroxidation, an ER stress response and macrophage activation in HCCs.
a, Quantification of lipid peroxidation in MycOE;NrasG12V;Cdkn2aARF–/– cells, upon 3 days of treatment with sorafenib, T0901317 or a combination thereof (by analysing the ratio of green and red fluorescence, values represent mean, ± SD, n = 3 cell cultures per condition, statistical significance was calculated using two-tailed Student´s t test, the analysis was repeated twice with similar results). b, Representative western blot analysis of uncleaved or cleaved Atf6, BiP/HSPA5, P-IRE1α (S724) and Xbp-1s in MycOE;NrasG12V;Cdkn2aARF–/– cells after 3 days of treatment with T0901317, sorafenib or a combination thereof (cropped blot images, n = 3 independent experiments). α-tubulin was used as a loading control. c, Representative western blot analysis of eIF2α, P-eIF2α (S51), GADD34, BiP/HSPA5 and P-IRE1α (S724) in MycOE;NrasG12V;Cdkn2aARF–/– cells after 3 days of treatment with T0901317, BI-882370 or a combination thereof (cropped blot images, n = 3 independent experiments). α-tubulin and Vinculin were used as loading controls. d, Representative western blot analysis of MLKL, P-MLKL (S345), RIPK3, P-RIPK3 (S232) and AIF (nuclear and cytoplasmic fraction) in MycOE;NrasG12V;Cdkn2aARF–/– cells after 3 days of treatment with T0901317, sorafenib or a combination thereof (cropped blot images, n = 3 independent experiments). Vinculin and Histone H3 were used as loading controls. e, FACS based analysis of myeloid cells in MycOE;NrasG12V HCCs upon treatment with T0901317/sorafenib or the corresponding monotherapies (3 d). f-h, Flow cytometry gating strategy. The following populations are identified: immature myeloid cells (iMC: CD11b+,Ly6G+/High,F4/80−, fraction 1), monocyte-macrophages (Mac-mono: CD11b+,Ly6G+,F4/80High, fraction 2) and Kupffer cells (Ku: CD11b+,Ly6G+,F4/80High, fraction 3). Monocyte and macrophages are further distinguished/labeled as Ly6C+ (fraction 2a) or Ly6C− (fraction 2b). Samples were gated on viable leukocytes by DAPI exclusion and doublets were excluded using height versus area dot plots. (shown are representative pseudocolor plots, n = 3 mice per group). i,j, Results of FACS analysis in MycOE;NrasG12V HCCs upon 3 days of treatment with T0901317/sorafenib, T0901317, sorafenib or carrier. Charts show the percentage mean ± SD of monocyte-macrophages (Mac−mono: CD11b+,Ly6G+,F/480+, i), Kupffer cells (Ku: CD11b+,Ly6G+,F4/80High, i) and immature myeloid cells (iMC: CD11b+, j) (n = 3 mice per group, statistical significance was calculated using two-tailed Student´s t test, the experiment was repeated with similar results). k, Treatment of MycOE;NrasG12V;Cdkn2aARF–/– cells for 4 days with T0901317 and/or the Bcl-2 inhibitor ABT-199 (quantification of viable cells by crystal violet staining, values represent mean ± SD, n = 3 independent experiments, statistical significance was calculated using two-tailed Student´s t test). Unprocessed images of the blots and numerical source data are provided as source data files.
Extended Data Fig. 7 Raf-1 stabilizes SCD1 protein in liver cancer.
a, Quantification of western blot analysis (shown in Fig. 6b) to quantify relative SCD1 expression in MycOE;NrasG12V;Cdkn2aARF–/– cells upon 3 days of treatment with sorafenib, T0901317 or a combination thereof (quantified with ImageJ, only samples from the same blot were compared, values represent mean ± SD, n = 6 independent experiments, statistical significance was calculated using two-tailed Student´s t test, α-tubulin was used as a loading control). b, Quantification of western blot analysis (shown in Fig. 6c) to quantify relative SCD1 expression in MycOE;NrasG12V;Cdkn2aARF–/– cells upon treatment with T0901317 (2 d) and sorafenib (1-3 h) (quantified with ImageJ, only samples from the same blot were compared, values represent mean ± SD, n = 6 independent experiments, statistical significance was calculated using two-tailed Student´s t test, α-tubulin was used as a loading control). c, Representative western blot analysis of SCD1 in HLE cells upon treatment with T0901317 (2 d) and sorafenib (1-3 h) (cropped blot images, n = 3 independent experiments). α-tubulin was used as a loading control. d-f, Representative western blot analysis of SCD1 in MycOE;NrasG12V;Cdkn2aARF–/– cells upon treatment with T0901317 (2 d) and the DFG-out RAF inhibitors RAF-265 (d) and RAF-709 (e) or the DFG-in DFG-in RAF inhibitor SB590885 (f) for 1 to 3 h (cropped blot images, n = 2 independent experiments). α-tubulin was used as a loading control. g, Representative western blot analysis of MEK and p-MEK1/2 (Ser217/221) in MycOE;NrasG12V;Cdkn2aARF–/– cells upon 6 h of treatment with sorafenib, BI-882370, dabrafenib or SB590885 (cropped blot images, n = 2 independent experiments). α-tubulin was used as a loading control. h, Representative western blot analysis of SCD1 in MycOE;NrasG12V;Cdkn2aARF–/–;Raf1WT cells upon treatment with T0901317 (2 d) and sorafenib for 1 to 3 h (cropped blot images, n = 2 independent experiments). α-tubulin was used as a loading control. i, Representative western blot analysis of Raf-1 in MycOE;NrasG12V;Cdkn2aARF–/–;shRaf1 or MycOE;NrasG12V;Cdkn2aARF–/–;shNC cells, 4 days upon treatment with doxycycline (cropped blot images, n = 2 independent experiments). j, Treatment of MycOE;NrasG12V;Cdkn2aARF–/– cells with carrier, T0901317, the MEK1/2 inhibitor PD0325901 or a combination thereof (quantification of viable cells by crystal violet staining, values represent mean ± SD, n = 3 independent experiments, statistical significance was calculated using two-tailed Student´s t test). Unprocessed images of the blots and numerical source data are provided as source data files.
Extended Data Fig. 8 A lipotoxic therapy did not induce adverse metabolic effects in murine livers.
a, Analysing potential liver damage in C57BL/6 wildtype mice upon a 3-week T0901317/sorafenib treatment. b, Liver-to-body ratio of mice upon a 3-week treatment with T0901317/sorafenib or carrier (values represent mean ± SD, n = 3 mice per group, statistical significance was calculated using two-tailed Student´s t test). c, Representative pictures of murine livers upon a 3-week treatment with T0901317/sorafenib or carrier that were stained with H+E, Oil red O, Ki67, cleaved caspase 3, TUNEL and Sirius red (n = 3 mice per group). TUNEL and cleaved caspase 3 positive control = Intraperitoneal injection of Jo2 antibody. Sirius red positive control = Bi-weekly intraperitoneal injections of CCl4 for 6 weeks. Ki67 positive control = MycOE;NrasG12V HCC. Scale bars, 100 µm. d, Analysing potential liver damage in C57BL/6 wildtype mice upon 6 months of T0901317/sorafenib treatment. e, Liver-to-body ratio of mice upon a 6-month treatment with T0901317/sorafenib or carrier (values represent mean ± SD, n = 6 mice per group, statistical significance was calculated using two-tailed Student´s t test). f, Representative pictures of murine livers upon a 6-month treatment with T0901317/sorafenib or carrier that were stained with H + E, Oil red O, Ki67, cleaved caspase 3, TUNEL and Sirius red (n = 6 mice per group). Scale bars, 100 µm. g,h, Weight development of mice during 2 weeks (g) or 6 months (h) of treatment with T0901317/sorafenib or carrier (values represent mean ± SD, n = 3 (g) or 6 (h) mice per group, statistical significance was calculated using two-tailed Student´s t test). Numerical source data are provided as source data files.
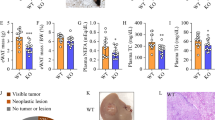
Extended Data Fig. 9 A lipotoxic therapy reduced NASH triggered HCC development and did not induce adverse metabolic effects in NASH livers.
a, Weight development of wildtype mice that were fed with choline deficient high fat diet (CD-HFD) or normal diet (ND) (values represent mean ± SD, n = 8 mice per group, statistical significance was calculated using two-tailed Student´s t test). b, Analysing tumor development and potential liver damage in NASH mice upon a 3-week T0901317/sorafenib treatment. c, Quantification of tumor nodules in mice that were fed for 12 months with CD-HFD and treated for 3 weeks with T0901317/sorafenib, sorafenib or carrier (values represent mean ± SD, n = 7 mice per group, statistical significance was calculated using two-tailed Student´s t test). d, Liver-to-body ratio of NASH mice upon a 3-week treatment with T0901317/sorafenib or carrier (values represent mean ± SD, n = 3 mice per group, statistical significance was calculated using two-tailed Student´s t test). e, Representative pictures of murine NASH livers upon a 3-week treatment with T0901317/sorafenib or carrier that were stained with H+E, Oil red O, Ki67, cleaved caspase 3, TUNEL and Sirius red (n = 3 mice per group). Scale bars, 100 µm. f, Weight development of NASH mice during treatment with T0901317/sorafenib or carrier (values represent mean ± SD, n = 3 mice per group, statistical significance was calculated using two-tailed Student´s t test). Numerical source data are provided as source data files.
Extended Data Fig. 10 Comparison of human HCC cells and weight development of tumor bearing mice.
a, Representative western blot analysis of Nrf2, Akt-1 and LXRα in different human HCC cells (cropped blot images, n = 3 independent experiments). β-actin was used as a loading control. b, LXRE reporter assay in different human HCC cells upon lentiviral infection of pL-LXRE-GFP (GFP measurements in individual cells; values represent mean ± SD, n = 5 independent experiments, statistical significance was calculated using two-tailed Student´s t test). c, Weight development of CB17.Cg-PrkdcscidLystbg/Crl mice upon subcapsular injection of HuH7 cells into the liver and treatment with T0901317/sorafenib or carrier (values represent mean ± SD, n = 6 mice per group statistical significance was calculated using two-tailed Student´s t test). Unprocessed images of the blots and numerical source data are provided as source data files.
Supplementary information
Supplementary Tables
Supplementary Tables 1–6.
Source data
Source Data Fig. 1
Numerical source data.
Source Data Fig. 2
Numerical source data.
Source Data Fig. 3
Numerical source data.
Source Data Fig. 4
Numerical source data.
Source Data Fig. 5
Numerical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Numerical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Numerical source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Fig. 8
Numerical source data.
Source Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 1
Numerical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Numerical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Numerical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Numerical source data.
Source Data Extended Data Fig. 5
Numerical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Numerical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Numerical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Numerical source data.
Source Data Extended Data Fig. 9
Numerical source data.
Source Data Extended Data Fig. 10
Numerical source data.
Source Data Extended Data Fig. 10
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Rudalska, R., Harbig, J., Snaebjornsson, M.T. et al. LXRα activation and Raf inhibition trigger lethal lipotoxicity in liver cancer. Nat Cancer 2, 201–217 (2021). https://doi.org/10.1038/s43018-020-00168-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-020-00168-3
This article is cited by
-
The role of DGAT1 and DGAT2 in regulating tumor cell growth and their potential clinical implications
Journal of Translational Medicine (2024)
-
Dysregulated cholesterol regulatory genes in hepatocellular carcinoma
European Journal of Medical Research (2023)
-
Advances in regulation and function of stearoyl-CoA desaturase 1 in cancer, from bench to bed
Science China Life Sciences (2023)
-
Exploiting lipotoxicity for the treatment of liver cancer
British Journal of Cancer (2021)
-
The immunological and metabolic landscape in primary and metastatic liver cancer
Nature Reviews Cancer (2021)