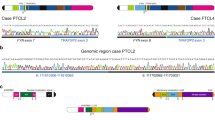
Abstract
Angioimmunoblastic T-cell lymphoma (AITL) and peripheral T-cell lymphoma not otherwise specified (PTCL, NOS) have poor prognosis and, in most cases, lack driver actionable targets for directed therapies. Here we identify FYN–TRAF3IP2 as a recurrent oncogenic gene fusion in AITL and PTCL, NOS tumors. Mechanistically, we show that FYN–TRAF3IP2 leads to aberrant NF-κB signaling downstream of T-cell antigen receptor activation. Consistent with a driver oncogenic role, FYN–TRAF3IP2 expression in hematopoietic progenitors induces NF-κB-driven T-cell transformation in mice and cooperates with loss of the Tet2 tumor suppressor in PTCL development. Moreover, abrogation of NF-κB signaling in FYN–TRAF3IP2-induced tumors with IκB kinase inhibitors delivers strong anti-lymphoma effects in vitro and in vivo. These results demonstrate an oncogenic and pharmacologically targetable role for FYN–TRAF3IP2 in PTCLs and call for the clinical testing of anti-NF-κB targeted therapies in these diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
DNA and RNA sequencing data that support the findings of this study have been deposited in the database of Genotypes and Phenotypes (dbGaP), (phs001962.v1.p1), and Gene Expression Omnibus (GEO), GSE138416. Previously published datasets that were reanalyzed during this study include RNA-seq data from individuals with AITL and PTCL, NOS in dbGaP (phs000689.v1.p1) and in the Sequence Read Archive (SRP029591), as well as RNA-seq data from mouse AITL with RhoA G17V in the GEO (GSE83918). We performed GSEA with gene sets available in the Molecular Signatures Database (MSigDB), https://www.gsea-msigdb.org/gsea/msigdb, and T. Gilmore’s NF-κB target database (Boston University; http://www.bu.edu/nf-kb/gene-resources/target-genes/). Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Vose, J., Armitage, J. & Weisenburger, D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J. Clin. Oncol. 26, 4124–4130 (2008).
Quivoron, C. et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell 20, 25–38 (2011).
Palomero, T. et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat. Genet. 46, 166–170 (2014).
Sakata-Yanagimoto, M. et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat. Genet. 46, 171–175 (2014).
Cairns, R. A. et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood 119, 1901–1903 (2012).
Wang, C. et al. IDH2R172 mutations define a unique subgroup of patients with angioimmunoblastic T-cell lymphoma. Blood 126, 1741–1752 (2015).
Couronne, L., Bastard, C. & Bernard, O. A. TET2 and DNMT3A mutations in human T-cell lymphoma. N. Engl. J. Med. 366, 95–96 (2012).
Yoo, H. Y. et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma. Nat. Genet. 46, 371–375 (2014).
Manso, R. et al. The RHOA G17V gene mutation occurs frequently in peripheral T-cell lymphoma and is associated with a characteristic molecular signature. Blood 123, 2893–2894 (2014).
Odejide, O. et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood 123, 1293–1296 (2014).
Abate, F. et al. Activating mutations and translocations in the guanine exchange factor VAV1 in peripheral T-cell lymphomas. Proc. Natl Acad. Sci. USA 114, 764–769 (2017).
Boddicker, R. L. et al. Integrated mate-pair and RNA sequencing identifies novel, targetable gene fusions in peripheral T-cell lymphoma. Blood 128, 1234–1245 (2016).
Rohr, J. et al. Recurrent activating mutations of CD28 in peripheral T-cell lymphomas. Leukemia 30, 1062–1070 (2016).
Vallois, D. et al. Activating mutations in genes related to TCR signaling in angioimmunoblastic and other follicular helper T-cell–derived lymphomas. Blood 128, 1490–1502 (2016).
Watatani, Y. et al. Molecular heterogeneity in peripheral T-cell lymphoma, not otherwise specified revealed by comprehensive genetic profiling. Leukemia 33, 2867–2883 (2019).
Attygalle, A. D., Feldman, A. L. & Dogan, A. ITK/SYK translocation in angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 37, 1456–1457 (2013).
Huang, Y. et al. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am. J. Surg. Pathol. 33, 682–690 (2009).
Streubel, B., Vinatzer, U., Willheim, M., Raderer, M. & Chott, A. Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia 20, 313–318 (2006).
Palacios, E. H. & Weiss, A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 23, 7990–8000 (2004).
Chang, S. H., Park, H. & Dong, C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J. Biol. Chem. 281, 35603–35607 (2006).
Li, X. et al. Act1, an NF-κB-activating protein. Proc. Natl Acad. Sci. USA 97, 10489–10493 (2000).
Qian, Y. et al. The adaptor Act1 is required for interleukin 17–dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 8, 247–256 (2007).
Gaffen, S. L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9, 556–567 (2009).
Ishigame, H. et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119 (2009).
Kuestner, R. E. et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol. 179, 5462–5473 (2007).
Wilcox, R. A. A three-signal model of T-cell lymphoma pathogenesis. Am. J. Hematol. 91, 113–122 (2016).
Chakraborty, A. K. & Weiss, A. Insights into the initiation of TCR signaling. Nat. Immunol. 15, 798–807 (2014).
Sato, I. et al. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J. Cell Sci. 122, 965–975 (2009).
Wolven, A., Okamura, H., Rosenblatt, Y. & Resh, M. D. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Mol. Biol. Cell 8, 1159–1173 (1997).
Meininger, I. & Krappmann, D. Lymphocyte signaling and activation by the CARMA1–BCL10–MALT1 signalosome. Biol. Chem. 397, 1315–1333 (2016).
Wang, D. et al. A requirement for CARMA1 in TCR-induced NF-κB activation. Nat. Immunol. 3, 830–835 (2002).
Fontan, L. et al. Specific covalent inhibition of MALT1 paracaspase suppresses B cell lymphoma growth. J. Clin. Invest. 128, 4397–4412 (2018).
Schlauderer, F. et al. Molecular architecture and regulation of BCL10–MALT1 filaments. Nat. Commun. 9, 4041 (2018).
Walsh, M. C., Lee, J. & Choi, Y. Tumor necrosis factor receptor-associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev. 266, 72–92 (2015).
Liu, C. et al. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci. Signal. 2, ra63 (2009).
Ryzhakov, G., Blazek, K. & Udalova, I. A. Evolution of vertebrate immunity: sequence and functional analysis of the SEFIR domain family member Act1. J. Mol. Evol. 72, 521–530 (2011).
Sonder, S. U. et al. IL-17-induced NF-κB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J. Biol. Chem. 286, 12881–12890 (2011).
Lemonnier, F. et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood 120, 1466–1469 (2012).
Chtanova, T. et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004).
de Leval, L. et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood 109, 4952–4963 (2007).
Saba, N. S. et al. Pathogenic role of B-cell receptor signaling and canonical NF-κB activation in mantle cell lymphoma. Blood 128, 82–92 (2016).
Awasthee, N. et al. Targeting IκB kinases for cancer therapy. Semin. Cancer Biol. 56, 12–24 (2019).
Burke, J. R. et al. BMS-345541 is a highly selective inhibitor of IκB kinase that binds at an allosteric site of the enzyme and blocks NF-κB-dependent transcription in mice. J. Biol. Chem. 278, 1450–1456 (2003).
Waelchli, R. et al. Design and preparation of 2-benzamido-pyrimidines as inhibitors of IKK. Bioorg. Med. Chem. Lett. 16, 108–112 (2006).
Lim, K. H., Yang, Y. & Staudt, L. M. Pathogenetic importance and therapeutic implications of NF-κB in lymphoid malignancies. Immunol. Rev. 246, 359–378 (2012).
Krappmann, D. & Vincendeau, M. Mechanisms of NF-κB deregulation in lymphoid malignancies. Semin. Cancer Biol. 39, 3–14 (2016).
Odqvist, L. et al. NIK controls classical and alternative NF-κB activation and is necessary for the survival of human T-cell lymphoma cells. Clin. Cancer Res. 19, 2319–2330 (2013).
Martínez-Delgado, B. et al. Differential expression of NF-κB pathway genes among peripheral T-cell lymphomas. Leukemia 19, 2254–2263 (2005).
Izban, K. F. et al. Constitutive expression of NF-κB is a characteristic feature of mycosis fungoides: implications for apoptosis resistance and pathogenesis. Hum. Pathol. 31, 1482–1490 (2000).
Fracchiolla, N. S. et al. Structural alterations of the NF-κB transcription factor lyt-10 in lymphoid malignancies. Oncogene 8, 2839–2845 (1993).
Derudder, E. et al. Identification and characterization of p100HB, a new mutant form of p100/NF-κB2. Biochem. Biophys. Res. Commun. 308, 744–749 (2003).
Mondragon, L. et al. GAPDH overexpression in the T cell lineage promotes angioimmunoblastic T cell lymphoma through an NF-κB-dependent mechanism. Cancer Cell 36, 268–287 (2019).
Cortes, J. R. et al. RHOA G17V induces T follicular helper cell specification and promotes lymphomagenesis. Cancer Cell 33, 259–273 (2018).
Ng, S. Y. et al. RhoA G17V is sufficient to induce autoimmunity and promotes T-cell lymphomagenesis in mice. Blood 132, 935–947 (2018).
Dierks, C. et al. The ITK–SYK fusion oncogene induces a T-cell lymphoproliferative disease in mice mimicking human disease. Cancer Res. 70, 6193–6204 (2010).
Pechloff, K. et al. The fusion kinase ITK–SYK mimics a T cell receptor signal and drives oncogenesis in conditional mouse models of peripheral T cell lymphoma. J. Exp. Med. 207, 1031–1044 (2010).
Schmidt-Supprian, M. et al. Mature T cells depend on signaling through the IKK complex. Immunity 19, 377–389 (2003).
Iyer, M. K., Chinnaiyan, A. M. & Maher, C. A. ChimeraScan: a tool for identifying chimeric transcription in sequencing data. Bioinformatics 27, 2903–2904 (2011).
Abate, F. et al. Pegasus: a comprehensive annotation and prediction tool for detection of driver gene fusions in cancer. BMC Syst. Biol. 8, 97 (2014).
Layer, R. M., Chiang, C., Quinlan, A. R. & Hall, I. M. LUMPY: a probabilistic framework for structural variant discovery. Genome Biol. 15, R84 (2014).
Kimbrel, E. A., Davis, T. N., Bradner, J. E. & Kung, A. L. In vivo pharmacodynamic imaging of proteasome inhibition. Mol. Imaging 8, 140–147 (2009).
Moran-Crusio, K. et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 20, 11–24 (2011).
Aghajani, K., Keerthivasan, S., Yu, Y. & Gounari, F. Generation of CD4CreERT2 transgenic mice to study development of peripheral CD4-T-cells. Genesis 50, 908–913 (2012).
Hsu, M. S. et al. TCR sequencing can identify and track glioma-infiltrating T cells after DC vaccination. Cancer Immunol. Res. 4, 412–418 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Acknowledgements
We thank R. Levine (Memorial Sloan Kettering Cancer Center) for kindly providing the Tet2fl/fl mouse line and the Digital Computational Pathology Laboratory in the Department of Pathology and Cell Biology at Columbia University Irving Medical Center. This work was supported by St. Baldrick’s Foundation (A.A.F.); NIH grants P30 CA013696 (Confocal and Specialized Microscopy Shared Resource, Molecular Pathology Shared Resource, Oncology Precision Therapeutics and Imaging Core (OPTIC), Flow Cytometry Shared Resource, Genomics Shared Resource, Herbert Irving Comprehensive Cancer Center), R01 CA197945 (T.P.), R35 CA210065 (A.A.F.), R01 CA185486 (R.R.), R01 CA179044 (R.R.), U54 CA121852 (R.R.) and F30 CA225052 (C.S.M.); the Stewart Foundation (R.R.); Leukemia & Lymphoma Society grants TRP-6507-17 (T.P.), TRP-6163-12 (A.A.F.) and Special Fellow Award 3395-20 (S.A.); and Spanish Ministerio de Ciencia, Innovación y Universidades grant RTI2018-094274-B-I00 (E.C.). E.C. is an Academia Researcher of the Institució Catalana de Recerca i Estudis Avançats (ICREA) of the Generalitat de Catalunya. J.R.C. is supported by a Lady Tata Memorial Trust fellowship.
Author information
Authors and Affiliations
Contributions
C.S.M. performed molecular biology, cellular and animal experiments and wrote the manuscript. C.R. performed molecular biology and cellular experiments and wrote the manuscript. J.R.C. designed and contributed to mouse in vivo pharmacological experiments and wrote the manuscript. C.S.M., S.A.Q., J.Z. and F.A. analyzed RNA-seq and whole-genome sequencing data. A.J.C. contributed to mouse in vivo pharmacological experiments. C.F. and S.A. performed molecular biology and cellular experiments. W.-H.W.L., C.R.S. and G.B. provided histopathological analysis of mouse tumors and analyzed data. G.B, C.R.S, E.C. and G.I. contributed clinical samples. R.R. supervised clinical sample RNA-seq and whole-genome sequencing analyses. T.P. and A.A.F. designed the study, supervised the research and wrote the manuscript with C.S.M.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Cancer thanks Kojo Elenitoba-Johnson, Daniel Krappmann and Tak W. Mak for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 FYN-TRAF3IP2 detection by RT-PCR and dideoxynucleotide sequencing.
a, Reverse-transcription PCR (RT-PCR) amplification results of an independent panel of 31 PTCL RNA samples spanning the region of FYN-TRAF3IP2 fusion breakpoint. b, DNA sequencing chromatograms of the RT-PCR amplicons generated in a.
Extended Data Fig. 2 RT-PCR analysis of FYN-TRAF3IP2 in B cell malignancies.
a, Reverse-transcription PCR (RT-PCR) amplification results of a panel of 92 B cell malignancies RNA samples spanning the region of FYN-TRAF3IP2 fusion breakpoint. + indicates a PTCL positive control sample, - indicates a PTCL negative control sample. No PCR products corresponding to the size of the specific FYN-TRAF3IP2 amplicon were detected. b, DNA sequencing chromatogram of the RT-PCR amplicons generated in a for the positive control PTCL sample and in sample 66 which showed a weak band slightly smaller than the specific FYN-TRAF3IP2 product. Lack of priming in this sample ruled out the presence of a specific FYN-TRAF3IP2 RT-PCR product in sample 66.
Extended Data Fig. 3 Expression and functional analysis of IL17 receptor and CARD 11 dependent signaling in T cells.
a, RNA expression level of IL17RC, essential for a functional IL17 receptor68, in T cells and monocytes represented from DICE (Database of Immune Cell Expression, Expression quantitative trait loci and Epigenomics). b, NF-κB-GFP reporter activity in transduced Jurkat cells after stimulation with 200 ng/mL IL17A. Results are reported as mean of technical replicate values (bar) from 1 independent experiment with individual values (white circles). c, Immunoblot analysis of JPM50.6 CARD11 knockout and JPM50.6 cells reconstituted by CARD11-HA expression. Expression levels were verified for each independent experiment. d, NF-κB-GFP reporter activity after stimulation with 25 nM of PMA or 20 ng/mL TNFα as in c. e, Western blot analysis of FYN-TRAF3IP2-V5 and BCL10 expression in Jurkat cells and Jurkat BCL10 knockout cells infected with empty vector or FYN-TRAF3IP2-V5 expressing lentiviruses; and RT-PCR analysis of FYN-TRAF3IP2-V5 mRNA in the same Jurkat BCL10 knockout cells infected with empty vector or FYN-TRAF3IP2-V5 expressing lentiviruses. * and ** indicate non-specific bands detected by the V5 antibody, while the arrowhead indicates the location of the specific FYN-TRAF3IP2-V5 band detected with the same antibody. Expression levels were verified for each independent experiment.
Extended Data Fig. 4 Generation and characterization of FYN-TRAF3IP2-induced mouse PTCLs.
a. We transduced hematopoietic progenitors from CD4 Cre-ERT2 Tet2fl/fl mice with bicistronic retroviruses driving the expression of GFP, FYN-TRAF3IP2-V5 and GFP or wild type TRAF3IP2-V5 and GFP. We intravenously transplanted the infected cells into lethally irradiated C57BL/6 recipient mice and treated them 8 weeks post-transplant with vehicle only or with tamoxifen to preserve or delete Tet2 in CD4+ T cells, respectively. These cohorts of mice were then immunized with sheep red blood cells every 4-5 weeks to induce peripheral T cell activation. b, Representative histological micrographs of hematoxylin-eosin stained liver, lung, and kidney of Tet2fl/fl or Tet2-/- FYN-TRAF3IP2-induced lymphoma-bearing animals and the GFP only control. Tissues from 3 mice per group showed similar results. Scale bar = 200 μm. c, Representative FACS plots of mononuclear cells collected from liver, lung, and kidney of FYN-TRAF3IP2-induced lymphoma-bearing animals, showing GFP+ CD4+ cell infiltration. d, Quantitative analysis of lymphoma liver infiltration in FYN-TRAF3IP2 Tet2fl/fl and FYN-TRAF3IP2 Tet2-/- tumors. Results are reported as mean of values (bar) ± standard deviation (error bar) with individual values (white circles), n = 5 tumors from 5 different mice per condition. The P value was calculated using two-tailed Student’s t-test. e, Representative flow cytometry analyses of PD1, ICOS, BCL6 and CXCR5 Tfh cell marker expression in CD4+ GFP+ spleen tumor cells compared to CD4+ GFP− non-tumor cells from the same spleen. f, Flow cytometry analysis of PD1 and CXCR5 Tfh cell marker expression in CD4+ GFP− non-tumor cells and CD4+ GFP+ tumor cells from a representative FYN-TRAF3IP2-induced lymphoma-bearing spleen. g, Heatmap representation of Tfh-associated marker expression in CD4+ naïve wild type T cells, FYN-TRAF3IP2-induced CD4+ GFP+ lymphoma cells and RHOA G17V Tet2-/- AITL-like mouse tumor cells53. h, GSEA enrichment plots of differentially expressed genes associated with FYN-TRAF3IP2-induced mouse lymphoma cells compared to wild type naïve CD4+ T cells. AITL geneset: top differentially upregulated genes in AITL compared with PTCL, NOS (fold change 1.5, p < 0.002)38. Tfh geneset: top 100 genes associated with Tfh cells37.
Extended Data Fig. 5 Analysis of the Rhoa G17V mutation in FYN-TRAIF3I2-induced mouse PTCL samples.
DNA chromatograms corresponding to the sequence of Rhoa exon 3 in DNA from tumor CD4+ T cells sorted from five independent FYN-TRAIF3I2-induced lymphomas. Highlighted area indicates the sequence corresponding to Rhoa codon 17 which is wild type (G17) in every case. No mutations were detected in these analyses.
Extended Data Fig. 6 Characterization of FYN-TRAF3IP2-induced mouse PTCLs: transplantability and transcriptional profiling.
a, Kaplan-Meier survival curve of n = 9 mice transplanted with cell suspension containing FYN-TRAF3IP2 GFP+ lymphoma infiltrate. b, Spleens of secondary recipients transplanted with FYN-TRAF3IP2-induced lymphoma infiltrate. c, Representative histological micrographs of H&E stained spleen, liver, and lung of lymphoma transplanted mice at the endpoint showing lymphoma infiltration. Tissues from 3 mice showed similar results. Scale bar = 400 μm. d, Representative FACS plot showing GFP+ CD4+ lymphoma infiltrates in spleens from diseased secondary recipients. e, Representative flow cytometry analyses of PD1, ICOS and BCL6 Tfh cell marker expression in CD4+ GFP+ spleen tumor cells compared to CD4+ GFP− non-tumor cells from the same spleen. f, Tcrb gene clonal analysis of three secondary Tet2-/- FYN-TRAF3IP2 GFP+ lymphomas illustrated by side-by-side representation of Tcrb sequence reads from primary and secondary Tet2-/- FYN-TRAF3IP2 GFP+ lymphomas, indicating the retention and expansion of specific lymphoma clone with unique Tcrb rearrangements after transplantation. g, RNAseq data of six independent FYN-TRAF3IP2-induced mouse CD4+ GFP+ lymphomas and wild type isogenic mouse naïve CD4+ T cells represented as heat map of the unselected top 100 differentially expressed genes between FYN-TRAF3IP2-induced tumors and wild type CD4+ naïve T cells (mouse genes without known human orthologues excluded; scale bar shows color-coded differential expression, with red indicating higher levels of expression and blue indicating lower levels of expression). h, Volcano plot of all differentially expressed genes as in g (P value <0.001 and |log2(fold change)|> 1 deemed significant). Blue, significantly downregulated genes. Red, significantly upregulated genes. The number of significantly downregulated or upregulated genes is indicated. i, Principal component analysis plot of RNAseq data as in g. j-k, GSEA analyses of differentially expressed genes associated with FYN-TRAF3IP2-induced mouse lymphomas based on the RNAseq data and the Hallmark signatures69 from MSigDB. The top 6 upregulated signatures j and top 6 downregulated signatures k are represented as bar graphs of normalized enrichment scores and P values.
Extended Data Fig. 7 IKK inhibitor treatment in normal T-cells.
a, Representative flow cytometry plot and quantification of the analysis of apoptosis in normal CD4+ T cells and CD4 FYN-TRAF3IP2 lymphoma cells treated with vehicle only (DMSO) or the IKK16 inhibitor. b, Representative flow cytometry plot and quantification of analysis of apoptosis in normal CD4+ T cells and CD4 FYN-TRAF3IP2 lymphoma cells treated with vehicle only (DMSO) or the BMS345541 IKK inhibitor. c, Representative flow cytometry plot and quantification of normal lymphocytes in spleen and lymph nodes of mice treated with vehicle only or with the BMS-345541 IKK inhibitor in vivo. Results are reported as mean of replicate values from n = 3 mice (bar) ± standard deviation (error bar) with individual values (black circles). P values were calculated using two-tailed Student’s t-test.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Supplementary Tables
Supplementary Tables 1–8.
Source data
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Unprocessed western blots.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed gels.
Source Data Extended Data Fig. 2
Unprocessed gels.
Source Data Extended Data Fig. 3
Unprocessed western blots and gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
About this article
Cite this article
Moon, C.S., Reglero, C., Cortes, J.R. et al. FYN–TRAF3IP2 induces NF-κB signaling-driven peripheral T-cell lymphoma. Nat Cancer 2, 98–113 (2021). https://doi.org/10.1038/s43018-020-00161-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-020-00161-w
This article is cited by
-
FYN: emerging biological roles and potential therapeutic targets in cancer
Journal of Translational Medicine (2023)
-
Advances in the expression and function of Fyn in different human tumors
Clinical and Translational Oncology (2023)
-
Role of Fyn in hematological malignancies
Journal of Cancer Research and Clinical Oncology (2023)
-
USP8 inhibition reshapes an inflamed tumor microenvironment that potentiates the immunotherapy
Nature Communications (2022)
-
Fusion transcripts FYN-TRAF3IP2 and KHDRBS1-LCK hijack T cell receptor signaling in peripheral T-cell lymphoma, not otherwise specified
Nature Communications (2021)