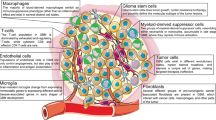
Abstract
Glioblastoma (GBM) is a devastating human malignancy. GBM stem-like cells (GSCs) drive tumor initiation and progression. Yet the molecular determinants defining GSCs in their native state in patients remain poorly understood. Here we used single-cell datasets and identified GSCs at the apex of the differentiation hierarchy of GBM. By reconstructing the GSCs’ regulatory network, we identified the YAP/TAZ coactivators as master regulators of this cell state, irrespectively of GBM subtypes. YAP/TAZ are required to install GSC properties in primary cells downstream of multiple oncogenic lesions and are required for tumor initiation and maintenance in vivo in different mouse and human GBM models. YAP/TAZ act as main roadblock of GSC differentiation, and their inhibition irreversibly locks differentiated GBM cells into a nontumorigenic state, preventing plasticity and regeneration of GSC-like cells. Thus, GSC identity is linked to a key molecular hub integrating genetics and microenvironmental inputs within the multifaceted biology of GBM.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All RNA-seq and microarray raw data generated for the present study, along with counts matrices and metadata for each sample, are publicly available in GEO under accession code GSE133471. The scRNA-seq data of primary glioblastoma samples from Darmanis et al. were downloaded as raw reads from GEO (GSE84465). The expression matrix and metadata of the Neftel dataset were downloaded from the Single Cell Portal of the Broad Institute (https://singlecell.broadinstitute.org/single_cell/study/SCP393/single-cell-rna-seq-of-adult-and-pediatric-glioblastoma#study-summary). Raw gene expression data (.CEL files) of the GBM TCGA cohort were downloaded from GEO (GSE83130). Raw gene expression (.CEL files) and clinical data of the REMBRANDT study were downloaded from GEO (GSE108474). BAM files of Ivy Atlas GBM samples were downloaded from the Anatomic Structures RNA-Seq repository of the Ivy Glioblastoma Atlas Project (http://glioblastoma.alleninstitute.org/rnaseq/bam.csv). Source data for Figs. 1e, 3b,e–f, 4c,f,g, 5a–f, 6b,f and Extended Data Figs. 1c, 2b,c, 3b, 4b, 6a–d, 7d, 8b, 9a,b and 10b,e,f have been provided with the paper. All other data supporting the findings of this study are available from the corresponding authors on reasonable request.
Code availability
All code used R 3.5.0 and publicly available packages cited in the paper. No custom functions were written for the analysis. STAR is available in GitHub (https://github.com/alexdobin/STAR). ARACNe-AP is available in GitHub (https://github.com/califano-lab/ARACNe-AP). HOMER is available at http://homer.ucsd.edu/homer/. MultiExperiment Viewer is available at http://mev.tm4.org.
References
Chen, J., McKay, R. M. & Parada, L. F. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 149, 36–47 (2012).
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004).
Galli, R. et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 64, 7011–7021 (2004).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
Gimple, R. C., Bhargava, S., Dixit, D. & Rich, J. N. Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 33, 591–609 (2019).
Pollard, S. M. et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell 4, 568–580 (2009).
Darmanis, S. et al. Single-cell RNA-seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 21, 1399–1410 (2017).
Neftel, C. et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178, 835–849 e821 (2019).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Pollen, A. A. et al. Molecular identity of human outer radial glia during cortical development. Cell 163, 55–67 (2015).
Chen, J. et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488, 522–526 (2012).
Lathia, J. D. et al. Integrin-α6 regulates glioblastoma stem cells. Cell Stem Cell 6, 421–432 (2010).
Bertolini, J. A. et al. Mapping the global chromatin connectivity network for Sox2 function in neural stem cell maintenance. Cell Stem Cell 24, 462–476 (2019).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Miroshnikova, Y. A. et al. Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol. 18, 1336–1345 (2016).
Hambardzumyan, D., Gutmann, D. H. & Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19, 20–27 (2016).
Lathia, J. D., Mack, S. C., Mulkearns-Hubert, E. E., Valentim, C. L. & Rich, J. N. Cancer stem cells in glioblastoma. Genes Dev. 29, 1203–1217 (2015).
Minata, M. et al. Phenotypic plasticity of invasive edge glioma stem-like cells in response to ionizing radiation. Cell Rep. 26, 1893–1905 (2019).
Laks, D. R. et al. Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem Cells 27, 980–987 (2009).
Pallini, R. et al. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin. Cancer Res. 14, 8205–8212 (2008).
Zeppernick, F. et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin. Cancer Res. 14, 123–129 (2008).
Brennan, C. W. et al. The somatic genomic landscape of glioblastoma. Cell 155, 462–477 (2013).
Gusev, Y. et al. The REMBRANDT study, a large collection of genomic data from brain cancer patients. Sci. Data 5, 180158 (2018).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Brennecke, P. et al. Accounting for technical noise in single-cell RNA-seq experiments. Nat. Methods 10, 1093–1095 (2013).
Grun, D., Kester, L. & van Oudenaarden, A. Validation of noise models for single-cell transcriptomics. Nat. Methods 11, 637–640 (2014).
Suva, M. L. et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell 157, 580–594 (2014).
Park, N. I. et al. ASCL1 reorganizes chromatin to direct neuronal fate and suppress tumorigenicity of glioblastoma stem cells. Cell Stem Cell 21, 411 (2017).
Breunig, J. J. et al. Ets factors regulate neural stem cell depletion and gliogenesis in ras pathway glioma. Cell Rep. 12, 258–271 (2015).
Paik, J. H. et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 5, 540–553 (2009).
Le Dreau, G. et al. E proteins sharpen neurogenesis by modulating proneural bHLH transcription factors’ activity in an E-box-dependent manner. eLife https://doi.org/10.7554/eLife.37267 (2018).
Kang, P. et al. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron 74, 79–94 (2012).
Mu, L. et al. SoxC transcription factors are required for neuronal differentiation in adult hippocampal neurogenesis. J. Neurosci. 32, 3067–3080 (2012).
Zanconato, F., Cordenonsi, M. & Piccolo, S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat. Rev. Cancer https://doi.org/10.1038/s41568-019-0168-y (2019).
Orr, B. A. et al. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J. Neuropathol. Exp. Neurol. 70, 568–577 (2011).
Tian, T. et al. TAZ promotes temozolomide resistance by upregulating MCL-1 in human glioma cells. Biochem. Biophys. Res. Commun. 463, 638–643 (2015).
Zanconato, F. et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 17, 1218–1227 (2015).
Zanconato, F., Cordenonsi, M. & Piccolo, S. YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803 (2016).
Koo, J. H. et al. Induction of AP-1 by YAP/TAZ contributes to cell proliferation and organ growth. Genes Dev. 34, 72–86 (2020).
Lan, X. et al. Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy. Nature 549, 227–232 (2017).
Puchalski, R. B. et al. An anatomic transcriptional atlas of human glioblastoma. Science 360, 660–663 (2018).
Friedmann-Morvinski, D. et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science 338, 1080–1084 (2012).
Dai, C. et al. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 15, 1913–1925 (2001).
Persson, A. I. et al. Non-stem cell origin for oligodendroglioma. Cancer Cell 18, 669–682 (2010).
Alcantara Llaguno, S. R. et al. Adult lineage-restricted CNS progenitors specify distinct glioblastoma subtypes. Cancer Cell 28, 429–440 (2015).
Galvao, R. P. et al. Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. PNAS 111, E4214–E4223 (2014).
Lee, J. H. et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 560, 243–247 (2018).
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 (2008).
Ganat, Y. M. et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J. Neurosci. 26, 8609–8621 (2006).
Louis, D. N., Ohgaki, H., Wiestler, O. D. & Cavenee, W. K. World Health Organization Histological Classification of Tumours of the Central Nervous System (International Agency for Research on Cancer, 2016).
Er, E. E. et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat. Cell Biol. 20, 966–978 (2018).
Zywitza, V., Misios, A., Bunatyan, L., Willnow, T. E. & Rajewsky, N. Single-cell transcriptomics characterizes cell types in the subventricular zone and uncovers molecular defects impairing adult neurogenesis. Cell Rep. 25, 2457–2469 (2018).
Caren, H. et al. Glioblastoma stem cells respond to differentiation cues but fail to undergo commitment and terminal cell-cycle arrest. Stem Cell Rep. 5, 829–842 (2015).
Auffinger, B. et al. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 21, 1119–1131 (2014).
Piccirillo, S. G. & Vescovi, A. L. Bone morphogenetic proteins regulate tumorigenicity in human glioblastoma stem cells. Ernst Schering Found. Symp. Proc. 5, 59–81 (2006).
Pistollato, F. et al. Molecular mechanisms of HIF-1α modulation induced by oxygen tension and BMP2 in glioblastoma derived cells. PLoS ONE 4, e6206 (2009).
Oh, T. et al. Immunocompetent murine models for the study of glioblastoma immunotherapy. J. Transl. Med. 12, 107 (2014).
Zanconato, F. et al. Transcriptional addiction in cancer cells is mediated by YAP/TAZ through BRD4. Nat. Med. 24, 1599–1610 (2018).
Miller, T. E. et al. Transcription elongation factors represent in vivo cancer dependencies in glioblastoma. Nature 547, 355–359 (2017).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Yao, Z. et al. A single-cell roadmap of lineage bifurcation in human ESC models of embryonic brain development. Cell Stem Cell 20, 120–134 (2017).
Nowakowski, T. J. et al. Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 358, 1318–1323 (2017).
Zhong, S. et al. A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature 555, 524–528 (2018).
Kanton, S. et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 574, 418–422 (2019).
Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Corces, M. R. et al. The chromatin accessibility landscape of primary human cancers. Science https://doi.org/10.1126/science.aav1898 (2018).
Lachmann, A., Giorgi, F. M., Lopez, G. & Califano, A. ARACNe-AP: gene network reverse engineering through adaptive partitioning inference of mutual information. Bioinformatics 32, 2233–2235 (2016).
Alvarez, M. J. et al. Functional characterization of somatic mutations in cancer using network-based inference of protein activity. Nat. Genet. 48, 838–847 (2016).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Cordenonsi, M. et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011).
Panciera, T. et al. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell 19, 725–737 (2016).
Zhang, N. et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19, 27–38 (2010).
Schonhuber, N. et al. A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat. Med. 20, 1340–1347 (2014).
Azzolin, L. et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 158, 157–170 (2014).
Schildge, S., Bohrer, C., Beck, K. & Schachtrup, C. Isolation and culture of mouse cortical astrocytes. J. Vis. Exp. https://doi.org/10.3791/50079 (2013).
Porcu, E. et al. BMP9 counteracts the tumorigenic and pro-angiogenic potential of glioblastoma. Cell Death Differ. 25, 1808–1822 (2018).
Totaro, A. et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat. Commun. 8, 15206 (2017).
Acknowledgements
We thank I. Verma, J. Massagué and L. Naldini for plasmids and colleagues sharing their plasmids through Addgene (M.-C. Hung, L. Pedersen, C. Counter, C. Cepko, K. Hochedlinger and M. Meyerson). We thank D.J. Pan, D. Saur, J. Siveke and P. Bonaldo for gifts of mice, G. Basso for HuTu cells, G. Zuccolotto for the GFP/Luc-expressing lentiviral construct, V. Barbieri for in vivo experiments, V. Guzzardo for histology, S. Bresolin for microarrays, G. Leo for TAZ IHC analysis and M. Forcato for comments. M. Castellan was supported by a FIRC-AIRC fellowship for Italy. O.R. is supported by Fondazione Umberto Veronesi (Post-Doctoral Fellowship 2020). The research leading to these results has received funding from AIRC 5×1000 2018 ‘Metastasis as mechanodisease’ (ID, 22759) grant to S.P.; from AIRC IG Grant 2019 (ID, 23307) to S.P.; from the Italian Ministry of Education, University and Research under a MIUR-FARE grant to S.P. and a MIUR-PRIN Bando 2017 grant to S.P. (cod. 2017HWTP2K); from the European Research Council under the European Union’s Horizon 2020 research and innovation program (DENOVOSTEM grant agreement No 670126) to S.P.
Author information
Authors and Affiliations
Contributions
M. Castellan performed most of the in vitro and in vivo experiments and contributed to writing. A.F. carried out the initial experiments of this study. A. Guarnieri and G.B. carried out experiments with HuTu cells. T.P. optimized mouse astrocyte isolation and infection. F.Z. contributed to manuscript preparation. F.Z., H.L.S., P.C. and A.C. optimized technical procedures critical for experiments in vivo and in vitro; M.F. performed histology and histopathological evaluations. E.P. and A.R. performed brain tumor experiments. O.R., A. Grilli and S.B. performed bioinformatic analyses. S.P. and M. Cordenonsi conceived the initial hypothesis and experimental design, organized the work and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Identification of the gene expression program of GSCs.
a, Single-cell differentiation trajectory of GBM cells reconstructed by Monocle2 using single-cell RNA-seq data of the indicated cell populations from primary GBM samples of the Darmanis dataset. b, Gene set enrichment analysis (GSEA) for association between the cell populations at the start and at the end of the pseudotime trajectory of the neoplastic cells of the Darmanis datasets (as depicted in Fig. 1c), and gene sets denoting the identity of specific cell types. Gene lists denoting early neural progenitor cells (RG: Radial Glia; oRG: outer Radial Glia; vRG: ventricular Radial Glia) or neural stem cells (NSC) are indicated in red; those identifying neurons, astrocytes or committed neuronal progenitors (OPC: Oligodendrocyte Progenitor Cells; INP: Intermediate Neuronal Progenitors) are, respectively, in blue, purple and blue-green colors; gene lists enriched in the putative GSC and DGC populations are highlighted in orange and in light blue, respectively. Signatures are available in Supplementary Table 1. GSEA calculated FDR adjusting for multiple comparisons; details of p-value and FDR calculation are described in the GSEA website (http://software.broadinstitute.org/gsea/index.jsp). Related to Fig. 1c. c, Log2 expression levels of the indicated oRG (top graphs), NSC and GSC (middle graphs) and INP markers (bottom graphs) in the subpopulations of neoplastic cells of the Darmanis dataset that are at the start (GSC, n = 221 cells) and at the end (DGC, n = 221 cells) of the pseudotime trajectory depicted in Fig. 1c. Data are presented as mean + s.d. p-values were determined by unpaired two-tailed t test. d, RNA velocities (arrows) of neoplastic cells of the Darmanis dataset projected in the space of the first two principal components. Red and blue dots are the cells that are at the start (GSC) and at the end (DGC) of the pseudotime trajectory depicted in Fig. 1c.
Extended Data Fig. 2 Validation of the G-STEM signature.
a-b, Violin plots showing the expression of the G-STEM signature (right panels in (b)) on the cells at the start (Low; red dots in the left panels in (b)) of the pseudotime trajectories (a) of patient-specific cohorts of the Darmanis dataset, vs. the neoplastic cells that are on the opposite ends of the same trajectories (High; blue dots in the left panels in (b)). The p-values were determined by two-tailed Mann-Whitney test. c, Violin plots showing the expression of the G-STEM signature (right panel) on the cells at the start (Low; red dots in the middle panel) of the pseudotime trajectory (left panel) of the sole neoplastic cells of the Darmanis dataset, vs. the cells that are on the opposite ends of the same trajectory (High; blue dots in the middle panel). The p-values were determined by two-tailed Mann-Whitney test.
Extended Data Fig. 3 Characterization of the G-STEM signature.
a, Graphs depicting the most significant GO terms emerging from the Gene Ontology analyses of the genes composing the G-STEM and the DGC signatures. The full lists of significant GO terms of both signatures are in Supplementary Table 3. b, Log2 expression levels of the indicated components of the G-STEM signature in the subpopulations of neoplastic cells of the Darmanis dataset that are at the start (GSC, n = 221 cells) and at the end (DGC, n = 221 cells) of the pseudotime trajectory depicted in Fig. 1c. Data are presented as mean + s.d. p-values were determined by unpaired two-tailed t test.
Extended Data Fig. 4 Validation of the G-STEM signature in large datasets of GBM patients.
a, Gene set enrichment analysis (GSEA) for association between the cell population at the start of the pseudotime trajectory of the neoplastic cells of the Neftel datasets (as depicted in Fig. 1e) vs. all the other neoplastic cells and gene sets denoting the identity of specific cell types. Abbreviations and color codes are as in Extended Data Fig. 1b. Signatures are available in Supplementary Table 1. GSEA calculated FDR adjusting for multiple comparisons; details of p-value and FDR calculation are described in the GSEA website (http://software.broadinstitute.org/gsea/index.jsp). Related to Fig. 1e. b, Violin plots showing the expression of the G-STEM signature (bottom panels) on the cells at the start of the pseudotime trajectory (GSC; red dots in the top panels) of small tumor cohorts of the Neftel dataset, pre-sorted according to the Proneural, Classical or Mesenchymal classification of GBMs, vs. all the other neoplastic cells of the same cohorts (NON GSC; light blue dots in the top panels) of the same dataset. The p-values were determined by two-tailed Mann-Whitney test. (c) Kaplan–Meier analysis representing the probability of survival in n = 541 GBM patients from the TCGA dataset (left panel), n = 210 GBM patients from the REMBRANDT dataset (middle panel), and n = 390 GBM patients carrying wild-type IDH1 from the TCGA dataset (right panel), stratified according to high or low GSC-signature. The p-value of the Log-rank (Mantel-Cox) test reflects the significance of the association between GSC-signature ‘low’ and longer survival. G-STEM expression is prognostic for the vast majority of GBM, that is IDH1-wild type tumors (93%, of those annotated in the TGCA dataset; n = 390 out of 419 IDH1-annotated samples).
Extended Data Fig. 5 A computational procedure to identify candidate TRs controlling the gene expression program of GSCs.
a, Overview of the experimental flow for inference of the master Transcriptional Regulators (TRs) of the GSC state using the Rhabdomant pipeline on the Darmanis sc-RNA-seq dataset of primary GBM samples. See Methods for details. b, List of candidate master Transcriptional Regulators (TRs) emerging from the analysis of the Darmanis dataset of scRNA-seq dataset with the Rhabdomant pipeline, ordered on the base of their normalized enrichment signal (NES). The Rhabdomant pipeline calculated FDR adjusting for multiple comparisons; see Methods for details about p-value and FDR calculation. The lists of candidate master TRs of the GSC and of the DGC state are highlighted in orange and in light blue, respectively. The most significant candidate master TRs of the GSC state are indicated in red.
Extended Data Fig. 6 YAP/TAZ are required for GSC maintenance in vivo.
a-c, Effects of YAP/TAZ knockout on the growth of established subcuteaneous GBM-like lesions. Transformed cells were obtained by dissociation of gliomaspheres obtained from HER2CA- (a), shNF1/shp53- (b) or KRasG12V/shp53- (c) transformed R26CAGCreERT2; Yapfl/fl; Tazfl/fl newborn mouse astroglial cells (as in Fig. 3), and then injected in NOD-SCID mice. When subcutaneous tumors reached approximately 0.5 cm of diameter, mice were either fed with Tamoxifen food to induce YAP/TAZ knockout (YAP/TAZ KO), or maintained under normal diet (YAP/TAZ wt). Graphs are growth curves of YAP/TAZ wt (KRasG12V/shp53-, n = 4 mice; HER2CA, n = 6 mice; shNF1/shp53, n = 5 mice) and YAP/TAZ KO (KRasG12V/shp53-, n = 4 mice; HER2CA, n = 4; shNF1/shp53, n = 8 mice) tumors (average volume ± s.e.m.). d,e, Effects of YAP/TAZ knockout in tumors derived from KRasG12V/shp53 gliomaspheres, following the experimental setup described in a-c. d, Dot plot for tumor weight at sacrifice (YAP/TAZ wt, n = 8; YAP/TAZ KO, n = 6). Mean ± s.e.m. of the distribution are also shown. p-value was calculated by unpaired two-tail t-test. e, Representative H&E stainings. Scale bar, 2.5 mm. N, necrotic area; *, Matrigel residue. f, Tabular results showing the number of NOD/SCID mice displaying subcutaneous tumor formation after injection of cells dissociated either from gliomaspheres derived from HER2CA-transformed primary newborn astroglial cells (Primary tumors), or from HER2CA-gliomaspheres derived from one of the Primary tumors (Secondary tumors).
Extended Data Fig. 7 Ex-vivo reprogramming of normal neural cells into GSC-like cells.
a, GFAP and SOX2 stainings (scale bars, 50 μm) of the mouse SVZ, representative of n = 3 mice. Nuclei were counterstained with DAPI. b,c, GFAP, NESTIN and SOX2 stainings (scale bars, 50 μm) in mouse newborn astroglial cells, representative of two independent experiments. d, Gliomaspheres emerging from newborn astroglial cell cultures transformed by the indicated oncogenes (P0 spheres) were dissociated to single cells and replated at clonal density for gliomasphere formation (P1 to P10 spheres). Results are representative of three experiments with n = 3 replicates each. Data are presented as scatter dot plots and bar graphs showing mean with s.d. e, Left panel: H&E staining of a lesion obtained after intracranial transplantation of shNf1/shp53-transformed astroglial cells. N, necrotic area. Scale bar, 2.5 mm. Middle panel: High magnification of the same tumor, showing large polynucleated cells (arrowheads). Right panel: TAZ IHC on the same tumor. Scale bars, 100 μm. Experiments were independently repeated on n = 10 mice, with similar results. f, H&E staining of subcutaneous tumors obtained by injecting cells dissociated from gliomaspheres carrying the indicated oncogenic lesions, representative of: KRasG12V/shp53, n = 4 tumors; HER2CA, n = 6 tumors; shNf1/shp53, n = 5 tumors. N, necrotic areas. Scale bars, 250 μm. g, Number of mice displaying tumor formation after injection of cells dissociated from KRasG12V/shp53-gliomaspheres at the indicated cell dilutions. h, Top, Schematic representation of the serial transplantation assay performed with HER2CA-transformed cells (see Methods for details). Bottom, H&E staining (scale bars, 2.5 mm) of tumors obtained after each round of transplantation, representative of n = 4 primary tumors, n = 8 secondary tumors and n = 4 tertiary tumors, respectively. Numbers of mice developing tumors per numbers of transplanted mice are indicated in each picture. i, GSEA curves of the G-STEM and the DGC signatures in KRasG12V/shp53-tumors compared to the astroglial cells from which they derive. Signatures are available in Supplementary Table 7.
Extended Data Fig. 8 Oncogenic insults activate YAP/TAZ in transformed primary astroglial cells.
a, Bright-field and fluorescent pictures (representative of n = 5 independent samples each) of newborn astroglial cells transduced with lentiviral vectors encoding for the YAP/TAZ reporter 8xGTIIC-RFP-DD52, and with lentiviral vectors encoding for the indicated oncogenes or, as negative control, with empty vector, as in Fig. 3b. Images were taken 4 days after inducing oncogenic reprogramming by incubating cells in NSC medium. Scale bars, 50 μm. b, Compendium of Fig. 3c. Efficiency of Yap and Taz downregulation in R26CAG-CreERT2; Yapfl/fl; Tazfl/fl mouse newborn astroglial cells treated with either vehicle (Control) or 4OH-TAM (YAP/TAZ KO), as measured by qRT-PCR (mean + s.d. of all independent samples of three experiments). p-values are calculated by two-way ANOVA with Sidak’s multiple comparisons.
Extended Data Fig. 9 YAP/TAZ are required for GSC maintenance in vitro.
a, Control experiment of Fig. 5a–e. Gliomaspheres derived from HER2CA-transformed Yapfl/fl; Tazfl/fl newborn astroglial cells, not expressing CREERT2, were treated with either ethanol (Vehicle) or 4OH-TAM (TAM). Panels are representative images (left; scale bar, 100 µm) and quantifications (right; mean ± s.d. of two independent experiments, each performed with two replicates) of the number of gliomaspheres/cm2 in vehicle versus 4OH-TAM-treated samples. p-values were determined by two-way ANOVA with Sidak’s multiple comparisons test. In the absence of CREERT2 expression, treatment with 4OH-TAM is inconsequential for gliomasphere formation, indicating that gliomasphere disaggregation shown in Fig. 4a–e is specifically caused by YAP/TAZ deletion. b, P2 gliomaspheres derived from R26CAG-CreERT2; Yapfl/fl; Tazfl/fl newborn astroglial cells transformed with the indicated oncogenes were dissociated to single cells and replated at clonal density for P3 gliomasphere formation in presence of ethanol (YAP/TAZ wt), or of 4OH-TAM to induce YAP/TAZ knockout (YAP/TAZ KO). Data are presented as scatter dot plots (n = 3 replicates each) and bar graphs showing mean with s.d. The p-values were calculated by unpaired two-tailed t-test.
Extended Data Fig. 10 YAP/TAZ are required for GBM initiation in vivo.
a-c, Immunocompromised mice were injected intracranially with KRasG12V/shp53-transformed Yapfl/fl;Tazfl/fl cells, also transduced with dual luciferase-GFP expression vectors. Control animals (n = 6) were injected with cells transduced with Ad-GFP, whereas YAP/TAZ KO animals (n = 5) were injected with cells transduced with Ad-Cre. a, Representative images of brain bioluminescence. b, Bioluminescence quantification shown as scatter dot plots and bar graphs showing mean with s.d; p-value was calculated by unpaired two-tailed t-test. c, Representative H&E stainings. Scale bars, 2.5 mm in left panels and 250 μm in the magnification shown on the right. Arrowheads highlight the presence of large, polynucleated cells. d-f, Immunocompromised mice were injected intracranially with HuTu13 cells transduced with dual luciferase-GFP expression vectors, and transfected with siCo (Control; n = 5) or siYAP/TAZ (YAP/TAZ depleted; n = 5). d, Representative images of brain bioluminescence. e, Bioluminescence quantification shown as scatter dot plots and bar graphs showing mean with s.d.; unpaired two-tailed t-test p-values are shown. f, Representative H&E stainings. Scale bars, 2.5 mm in left panels and 250 μm in the magnification shown on the right. ‘N’ indicates necrosis. g-i, CT2A cells were transduced with dual luciferase-GFP expression vectors and injected intracranially in syngeneic mice. Control animals (n = 5) were injected with cells expressing anti-GFP shRNA, whereas YAP/TAZ-depleted animals (n = 5) were injected with cells expressing doxycycline-inducible YAP and TAZ shRNAs. g, Representative brain bioluminescences at one day and 14 days after injection. h, Bioluminescence quantification at three different time points shown as scatter dot plots and bar graphs showing mean with s.d.; unpaired two-tailed t-test p-values are shown. i, Representative H&E stainings. Scale bars, 2.5 mm in left panels and 250 μm in the magnification shown on the right. N, necrotic areas. j, GFP and TUJ1 stainings in sections from YAP/TAZ-wt and YAP/TAZ-KO subcutaneous shNF1/shp53-induced tumors (representative of n = 3 independent samples each). Scale bars, 50 µm.
Supplementary information
Supplementary Tables
Supplementary Tables 1–16
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Uncropped western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Castellan, M., Guarnieri, A., Fujimura, A. et al. Single-cell analyses reveal YAP/TAZ as regulators of stemness and cell plasticity in glioblastoma. Nat Cancer 2, 174–188 (2021). https://doi.org/10.1038/s43018-020-00150-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-020-00150-z
This article is cited by
-
Control of stem cell renewal and fate by YAP and TAZ
Nature Reviews Molecular Cell Biology (2023)
-
Cellular senescence in malignant cells promotes tumor progression in mouse and patient Glioblastoma
Nature Communications (2023)
-
Cell signaling activation and extracellular matrix remodeling underpin glioma tumor microenvironment heterogeneity and organization
Cellular Oncology (2023)
-
Temporal and spatial stability of the EM/PM molecular subtypes in adult diffuse glioma
Frontiers of Medicine (2023)
-
Metabolic modeling-based drug repurposing in Glioblastoma
Scientific Reports (2022)