Abstract
Human leukocyte antigen (HLA)-B has been recognized as a major determinant of discrepancies in disease outcomes, and recent evidence indicates a role in immune checkpoint blockade (ICB) efficacy. The B44 supertype, which features an electropositive binding pocket that preferentially displays peptides with negatively charged amino acid anchors, is associated with improved survival in ICB-treated melanoma. Yet this effect was not seen in ICB-treated non-small-cell lung cancer (NSCLC). Here we show that mutations leading to glutamic acid substitutions occur more often in melanoma than NSCLC based on mutational landscape. We additionally show stratifying B44 based on the presence of somatic mutations that lead to negatively charged glutamic acid anchors identifies patients with NSCLC with an ICB benefit similar to that seen in melanoma. We anticipate these findings could improve assessment of HLA-related outcomes and prediction of ICB benefit in those with B44, representing approximately half of the world’s population.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
WES data that support the findings of this study have been deposited in the database of Genotypes and Phenotypes (dbGaP) under accession code phs002244, which can be accessed with a usage agreement for nonprofit cancer-related research. WES data from L06, L13, L14, L21 and L26 are available from dbGaP accession code phs000980. Previously published datasets that were reanalyzed here are available under accession codes phs000452, phs000980, phs00694, phs001041, phs001565 and SRP067938. The LUAD, LUSC and melanoma data were derived from the TCGA Research Network at http://cancergenome.nih.gov/. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
The code used to support the findings of this study is publicly available at https://github.com/garon-lab/hlab44/.
References
Dausset, J. The major histocompatibility complex in man. Science 213, 1469–1474 (1981).
Algarra, I., Collado, A. & Garrido, F. Altered MHC class I antigens in tumors. Int. J. Clin. Lab. Res. 27, 95–102 (1997).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Sidney, J. et al. Several HLA alleles share overlapping peptide specificities. J. Immunol. 154, 247–259 (1995).
Sette, A. & Sidney, J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 50, 201–212 (1999).
Sidney, J., Peters, B., Frahm, N., Brander, C. & Sette, A. HLA class I supertypes: a revised and updated classification. BMC Immunol 9, 1 (2008).
Chowell, D. et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy.Science 359, 582–587 (2017).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Negrao, M. V. et al. PD-L1 expression, tumor mutational burden and cancer gene mutations are stronger predictors of benefit from immune checkpoint blockade than HLA class I genotype in non-small cell lung cancer.J. Thoracic Oncol. 14, 1021–1031 (2019).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 378, 2093–2104 (2018).
Chowell, D. et al. Evolutionary divergence of HLA class I genotype impacts efficacy of cancer immunotherapy. Nat. Med. 25, 1715–1720 (2019).
DiBrino, M. et al. Identification of the peptide binding motif for HLA-B44, one of the most common HLA-B alleles in the caucasian population. Biochemistry 34, 10130–10138 (1995).
Rammensee, H. G., Friede, T. & Stevanoviic, S. MHC ligands and peptide motifs: first listing. Immunogenetics 41, 178–228 (1995).
Sinha, N. & Smith-Gill, S. J. Electrostatics in protein binding and function. Curr. Protein Pept. Sci. 3, 601–614 (2002).
Macdonald, W. A. et al. A naturally selected dimorphism within the HLA-B44 supertype alters class I structure, peptide repertoire, and T cell recognition. J. Exp. Med. 198, 679–691 (2003).
Hodis, E. et al. A landscape of driver mutations in melanoma. Cell 150, 251–263 (2012).
Rizvi, N. A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Miao, D. et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat. Genet. 50, 1271–1281 (2018).
Cancer Genome Atlas Research, N. et al. The Cancer Genome Atlas pan-cancer analysis project. Nat. Genet. 45, 1113–1120 (2013).
Vitkup, D., Sander, C. & Church, G. M. The amino-acid mutational spectrum of human genetic disease. Genome Biol. 4, R72 (2003).
Zuckerkandl, E. & Pauling, L. Molecules as documents of evolutionary history. J. Theor. Biol. 8, 357–366 (1965).
Zhang, J. Rates of conservative and radical nonsynonymous nucleotide substitutions in mammalian nuclear genes. J. Mol. Evol. 50, 56–68 (2000).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Duan, F. et al. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J. Exp. Med. 211, 2231–2248 (2014).
Teku, G. N. & Vihinen, M. Pan-cancer analysis of neoepitopes. Sci. Rep. 8, 12735 (2018).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Herbst, R. S. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387, 1540–1550 (2016).
Ribas, A. et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. J. Am. Med. Assoc. 315, 1600–1609 (2016).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Liu, C. et al. ATHLATES: accurate typing of human leukocyte antigen through exome sequencing. Nucleic Acids Res. 41, e142 (2013).
DePristo, M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6, 80–92 (2012).
Zerbino, D. R. et al. Ensembl 2018. Nucleic Acids Res. 46, D754–D761 (2018).
Hundal, J. et al. pVAC-Seq: A genome-guided in silico approach to identifying tumor neoantigens. Genome Med. 8, 11 (2016).
Vita, R. et al. The immune epitope database (IEDB): 2018 update. Nucleic Acids Res. 47, 339–343 (2018).
Grimsley, G. R., Scholtz, J. M. & Pace, C. N. A summary of the measured pK values of the ionizable groups in folded proteins. Protein Sci. 18, 247–251 (2009).
Jencks W. P. & Regenstein J. in Handbook of Biochemistry and Molecular Biology 4th edn. (eds Lundblad, R. L. & MacDonald, F. M.) 595–635 (CRC Press, 2010).
Kessler, J. H. et al. Competition-based cellular peptide binding assay for HLA class I.Curr. Protoc. Immunol. 61, 18.12.1–18.12.15 (2004).
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 (2008).
Rosner, B., Glynn, R. J. & Lee, M. L. Extension of the rank sum test for clustered data: two-group comparisons with group membership defined at the subunit level. Biometrics 62, 1251–1259 (2006).
Acknowledgements
We thank N. Volkes and M. Negrao as well as D. Wu, S. Tang, C. Kravit and P. Petrousis for their assistance in this project. The results here are in part based on data generated by the TCGA Research Network: https://www.cancer.gov/tcga. This work was funded by E.B.G.’s grant NIH-NCI R01 CA28403, the Cancer Center Support grant P30 CA016042 and grant NIH-NCATS UL1TR001881. A.L.C. was funded by a Specialty Training and Advanced Research Award. Z.N. was funded by USHHS Ruth L. Kirschstein Institutional National Research Service Award no. T32 CA009120. A.L. was funded by grant NIH-NCI K08 CA245249-01A1 and a LUNGevity 2019 Career Development Award. A.A.T.B. was funded by grant NIH-NCI R01 CA226079. A.R. was funded by the Parker Institute for Cancer Immunotherapy, NIH-NCI grants R35 CA197633 and P01 CA244118, and the Ressler Family Fund. S.M.G. is funded by grant NIH-NCI IU01CA196408. E.B.G. is also funded by grant CLIN-10784.
Author information
Authors and Affiliations
Contributions
A.L.C., M.R. and E.B.G. contributed substantially to the conception and design of the work. A.L.C., J.G., H.Y.L., T.G., G.S., C.M.F., N.H., J.Z., J.C., B.B., W.O.A., D.L., Z.N., A.L., J.W.G., D.E., A.A.T.B., A.R., S.M.D., M.R. and E.B.G. contributed to data acquisition, analysis and interpretation. A.L.C. and E.B.G. drafted the paper. A.L.C., T.G., J.W.G., A.A.T.B., A.R., S.M.D., M.R. and E.B.G. contributed substantially to article revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
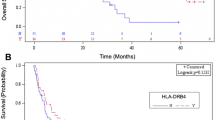
Extended Data Fig. 1 NSCLC survival based on B44 supertype subset.
OS – overall survival, PFS – progression-free survival. Survival estimated using the Kaplan-Meier method and compared with a non-parametric log-rank test. Dashed lines represent median. B44sub – B44 subset including HLA-B*18:01, B*44:02, B*44:03, B*44:05, B*50:017 (red, N = 27 patients). Other B4 supertype patients grouped with B44sub-absent (blue, N = 38 patients). Citation numbering based on manuscript references. a, Patients with at least one B44 subset allele had a median OS 7.6 months (95% CI 3.2 – 18.4) vs 18.6 months (95% CI 9.2 – 34.1), P=0.005. b, Patients with at least one B44 subset allele had a median PFS 2.1 months (95% CI 1.8 – 4.9) vs 8.8 months (95% CI 5.1 – 14.5), P=0.002.
Extended Data Fig. 2 Observed amino acid substitution profiles comparing UCLA NSCLC and melanoma cohorts.
Amino acids represented by standard single letter notation. Heatmaps represent average proportions of amino acid substitutions with wildtype amino acids on the x-axis and substituted amino acid on the y-axis. In panels a and b, black corresponds to rare substitutions while lighter colors represent enrichment. In panel c, orange corresponds to substitutions seen equally in NSCLC and melanoma, darker colors represent substitutions seen more commonly in melanoma, whiter colors those seen more commonly in NSCLC. See Methods for additional details. a, Observed NSCLC profile (N = 38 patients). b, Observed melanoma profile (N = 14 patients). c, Difference in observed NSCLC vs melanoma profile (NSCLC-MEL) supports decreased p.G>E in NSCLC relative to melanoma.
Extended Data Fig. 3 Correlation of DNA mutations, glutamic acid substitutions, and motif neoepitopes.
MEL – melanoma, NSCLC – non-small cell lung cancer, g. – genomic, A – adenine, C – cytosine, G – guanine, T – thymine. Typical scatter plots depicted with line of best fit calculated by least square method, 95% confidence interval shown in gray. a-d: Mutations that can result in radical glutamic acid substitutions (g.A>G, g.T>A, g.G>A, and g.C>A) are pictured. Each point represents a patient (NSCLC N = 38 patients, MEL N = 14 patients). Glutamic acid and mutation proportions calculated as proportion of all radical mutations. e-g: Correlation of glutamic acid substitutions to glutamic acid neoepitopes and B44 motif neoepitopes in NSCLC B44 patients (N=21). Each point represents the number of substitutions/neoepitopes for each B44 patient. All predicted neoepitopes had an IC50 ≤ 500 nM. a, Correlation of the proportion of g.A>G mutations to proportion of radical glutamic acid substitutions, Pearson correlation coefficient 0.03, P=0.85 (that is, correlation not similar between histologies). b, Correlation of the proportion of g.T>A mutations to proportion of radical glutamic acid substitutions, Pearson correlation coefficient -0.20, P=0.16. c, Correlation of the proportion of g.G>A mutations to proportion of radical glutamic acid substitutions, Pearson correlation coefficient 0.60, P<0.001. d, Correlation of the proportion of g.C>A mutations to proportion of radical glutamic acid substitutions, Pearson correlation coefficient -0.61, P<0.001. e, Correlation of the number of glutamic acid radical substitutions with the number of glutamic acid neoepitopes, Pearson correlation coefficient 0.56, P=0.009. f, Correlation of the number of neoepitopes including glutamic acid with the number of motif neoepitopes, Pearson correlation coefficient 0.89, P<0.001 (removing outlier motif neoepitopes=7, R=0.68, P=0.001). g, Correlation of the number of glutamic acid radical substitutions with the number of motif neoepitopes, Pearson correlation coefficient 0.42, P=0.06.
Extended Data Fig. 4 Predicted differences of half-maximal inhibitory concentrations of NSCLC motif neoepitopes in non-B44 HLA supertypes.
Boxplots summarize distribution of points with solid lines representing the median proportion (center), boxplot boundaries define the 25th and 75th percentiles, whisker boundaries define the minima and maxima. Black points appear next to outliers greater than 1.5 times the interquartile range. Each point represents a neoepitope prediction. Note HLA alleles with undefined B-pockets were not included. Motif neoepitopes require a radical substitution in the anchor position with a known C-terminus (see Methods). All predicted neoepitopes had an IC50 ≤ 500 nM. a, Predicted B07 neoepitope IC50 differences based on mutation to motif (present N = 32 predicted neoepitopes, absent N = 1566 predicted neoepitopes), Wilcoxon test of difference P<0.001. b, Predicted B27 neoepitope IC50 differences based on mutation to motif (present N = 5 predicted neoepitopes, absent N = 76 predicted neoepitopes), Wilcoxon test of difference P=0.019. c, Predicted B58 neoepitope IC50 differences based on mutation to motif (present N = 12 predicted neoepitopes, absent N = 310 predicted neoepitopes), Wilcoxon test of P<0.001. d, Predicted B62 neoepitope IC50 differences based on mutation to motif (present N = 34 predicted neoepitopes, absent N = 680 predicted neoepitopes), Wilcoxon test of difference P<0.001.
Extended Data Fig. 5 NSCLC overall and progression-free survival based on the presence of motif neoepitopes in all supertypes.
Survival estimated using the Kaplan-Meier method and compared between groups with a non-parametric log-rank test. Dashed lines represent medians. Motif neoepitopes required a radical substitution in the anchor position with a known C-terminus (see Methods), survival curves depicted in blue. The survival curves of those without motif neoepitopes are depicted in red. a, Patients with at least one motif neoepitope (N = 28 patients) had a median OS of 12.6 vs 5.2 months in those without (N = 10 patients), P=0.013. b, Patients with at least one motif neoepitope (N = 28 patients) had a median PFS of 5.6 vs 6.3 months in those without (N = 10 patients), P=0.120.
Extended Data Fig. 6 NSCLC survival based on supertype-specific motif neoepitopes.
Survival estimated using the Kaplan-Meier method and compared among supertypes with a non-parametric log-rank test. Dashed lines represent medians. Motif neoepitopes required a radical substitution in the anchor position with a known C-terminus (see Methods), survival curves depicted in blue. The survival curves of those without motif neoepitopes are depicted in red. a, UCLA B27 cohort, patients with motif neoepitopes (N = 3 patients) had a median OS not reached vs 11.5 months without (N = 2 patients) (HR cannot be calculated), P=0.039. b, UCLA B27 cohort, patients with motif neoepitopes (N = 3 patients) had a median PFS of 45.3 vs 8.2 months without (N = 2 patients) (HR 0.18), P=0.062. c, UCLA B44 cohort, patients with motif neoepitopes (N = 14 patients) had a median OS of 18.2 vs 7.0 months without (N = 7 patients) (HR 0.38), P=0.038. d, UCLA B44 cohort, patients with motif neoepitopes (N = 14 patients) had a median PFS of 8.7 vs 3.5 months without (N = 7 patients) (HR 0.40), P=0.072. e, UCLA B07 cohort, patients with motif neoepitopes (N = 15 patients) had a median PFS of 10.2 vs 10.9 months without (N = 4 patients), P=0.33. f, UCLA B58 cohort, patients with motif neoepitopes (N = 3 patients) had a median PFS of 10.2 vs 10.9 months without (N = 1 patient), P=0.92. g, UCLA B62 cohort, patients with motif neoepitopes (N = 7 patients) had a median PFS of 5.5 vs 6.2 months without (N = 1 patient), P=0.89.
Extended Data Fig. 7 Survival of cohorts based on supertype and motif neoepitopes.
Survival estimated using the Kaplan-Meier method. Survival curves of B27-(motif) absent patients are depicted in red, B27-motif (present) in blue, B27/B44-motif (present) in green, B44-(motif) absent in dark purple, B44-motif (present) in orange, other in light purple. Note given the rarity of B27/B44-motif absent patients (none in UCLA and DF-melanoma cohorts), a separate category was not created and these patient(s) were grouped as B44-(motif) absent. a, UCLA NSCLC cohort overall survival. Median survival times, longest to shortest (months): B27-motif present (not reached), B27/B44-motif present (18.7), other (18.2), B44-motif present (16.2), B44-motif absent (11.6), B27-motif absent (11.5). b, UCLA NSCLC cohort progression-free survival. Median survival times, longest to shortest (months): B27/B44-motif present (49.5), B27-motif present (44.7), other (10.6), B27-motif absent (8.2), B44-motif present (4.9), B44-motif absent (2.0). c, DF-NSCLC cohort overall survival. Median survival times, longest to shortest (months): B27/B44-motif present (not reached), B27-motif present (not reached), B44-motif present (not reached), B44-motif absent (not reached), other (13.4), B27-motif absent (8.4). Note 2 B27/B44-motif absent patients grouped with B44-motif absent (this group did not exist in other cohorts). d, DF-NSCLC cohort progression-free survival. Median survival times, longest to shortest (months): B27/B44-motif present (32.8), B27-motif present (not reached), B44-motif present (14.5), B44-motif absent (4.22), B27-motif absent (4.2), other (2.8). Note 2 B27/B44-motif absent patients grouped with B44-motif absent (this group did not exist in other cohorts). Not all patients had censoring data available leading to fewer patients in each category compared to DF-NSCLC OS. e, DF-melanoma cohort overall survival. Median survival times, longest to shortest (months): B27/B44-motif present (48.6), B44-motif present (34.5), B27-motif present (26.3), B44-motif absent (23.8), other (10.3), B27-motif absent (5.3). f, DF-melanoma cohort progression-free survival. Median survival times, longest to shortest (months): B44-motif present (19.6), B27-motif present (15.0), B27/B44-motif present (11.0), B27-motif absent (3.7), other (3.5), B44-motif absent (3.3).
Supplementary information
Supplementary Information
Supplementary Tables 1–7.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
About this article
Cite this article
Cummings, A.L., Gukasyan, J., Lu, H.Y. et al. Mutational landscape influences immunotherapy outcomes among patients with non-small-cell lung cancer with human leukocyte antigen supertype B44. Nat Cancer 1, 1167–1175 (2020). https://doi.org/10.1038/s43018-020-00140-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-020-00140-1
This article is cited by
-
Antigen presentation in cancer — mechanisms and clinical implications for immunotherapy
Nature Reviews Clinical Oncology (2023)
-
HLA-B*44 and the Bw4-80T motif are associated with poor outcome of relapse-preventive immunotherapy in acute myeloid leukemia
Cancer Immunology, Immunotherapy (2023)
-
Comparison of the tumor immune microenvironment and checkpoint blockade biomarkers between stage III and IV non-small cell lung cancer
Cancer Immunology, Immunotherapy (2023)
-
Functional landscapes of POLE and POLD1 mutations in checkpoint blockade-dependent antitumor immunity
Nature Genetics (2022)
-
Mutagenic exposures shape immunotherapy responses
Nature Cancer (2020)