Abstract
Coronavirus disease 2019 (COVID-19) and its causative virus, SARS-CoV-2, pose considerable challenges for the management of oncology patients. COVID-19 presents as a particularly severe respiratory and systemic infection in aging and immunosuppressed individuals, including patients with cancer. Moreover, severe COVID-19 is linked to an inflammatory burst and lymphopenia, which may aggravate cancer prognosis. Here we discuss why those with cancer are at higher risk of severe COVID-19, describe immune responses that confer protective or adverse reactions to this disease and indicate which antineoplastic therapies may either increase COVID-19 vulnerability or have a dual therapeutic effect on cancer and COVID-19.
Similar content being viewed by others
Main
After several local epidemics caused by coronaviruses (CoVs) in recent years, namely severe acute respiratory syndrome CoV (SARS-CoV) in 2002 and Middle East respiratory syndrome CoV (MERS-CoV) in 2012 and 2015, a novel virus, SARS-CoV-2, emerged at the end of 2019 and spread rapidly throughout the world to cause the pandemic known as COVID-191,2. 22 million cases of COVID-19 and over 780,000 deaths had been reported worldwide by mid-August 2020, with a mortality rate that remains elusive due to uncertainty about the true number of infections. Mechanistically, infection by either SARS-CoV or SARS-CoV-2 involves the action of the virus spike protein (S), which engages angiotensin-converting enzyme 2 (ACE2) as the entry receptor on the surface of target host cells3, and the cooption of the cellular serine protease TMPRSS2, a member of the type II transmembrane serine protease family of proteins, which are implicated in both cancer and viral infections4,5, for S protein priming6,7. The ACE2 receptor is highly expressed in lung alveolar type 2 cells, but is also present on endothelial and smooth muscle cells in various organs, including the heart, liver, kidney and digestive tract8. Although a substantial fraction of SARS-CoV-2-positive individuals are asymptomatic or paucisymptomatic carriers (the latter experiencing nonspecific symptoms similar to those of the common cold and sometimes gastroenteritis), severe COVID-19, which develops mostly in individuals with comorbidities, leads to acute respiratory failure, often associated with a cytokine storm, a prothrombotic immunopathology and profound lymphopenia, culminating in multiple organ dysfunction and death9,10,11,12.
Patients with cancer were considered more susceptible to SARS-CoV-2 infection than individuals without cancer not only because of age, given that cancer incidence is strongly linked to advancing age, but also because of the high prevalence of cancer risk factors also associated with COVID-19—in particular, thoracic computed tomography (CT) scan abnormalities and smoking, along with cancer-associated metabolic disorders such as diabetes and hypertension—as well as the side effects of chemotherapy that might aggravate COVID-19, including arterial hypertension, cardiomyopathy, systemic immunosuppression13 and accelerated cellular senescence14,15. Several reports have addressed the prevalence of patients with a clinical history of cancer in French, Chinese and Italian populations tested for SARS-CoV-2 infection16,17,18,19. One study of 84,246 consecutive individuals tested for SARS-CoV-2 from the Veneto region of Italy found that 5.7% had previously been diagnosed with cancer and, among those positive for SARS-CoV-2, 7.8% had a cancer diagnosis. Hence, the authors concluded that prevalence of cancer was not associated with risk of infection16. In contrast, two earlier studies reported an increased incidence of COVID-19 in patients diagnosed with cancer in China17,18. In the first report, patients with tumors were older and had a history of smoking or dyspnea or severe baseline CT scan manifestations, with 28% having been diagnosed with lung carcinoma17. In the second study, of 1,524 patients with cancer from a tertiary cancer center in China, the relative prevalence of COVID-19 was twice as high as in the general population18. Differences between the Italy-based studies versus China-based studies in the demographic profiles of their patient populations, such as a larger proportion of older males participating in the latter, may account for these apparent discrepancies.
The main risk factors of severe COVID-19 in the general population are gender (male/female sex ratio, 1.65:1), advanced age (median age >60), obesity and diseases such as congestive heart failure, coronary heart disease, diabetes, hypertension, hyperlipidemia and cancer9. Race and ethnicity are also associated with COVID-19 risk, with Black and Hispanic people being disproportionately affected compared with white people9. In contrast to the incidence, COVID-19 severity was found to increase when associated with cancer across studies and geographical sites, including France, China, the USA and Italy16,20,21. For instance, the study from the Veneto region of Italy reported a higher percentage of COVID-19-related hospitalizations (56% versus 34%) and deaths (14% versus 4%) among patients with a history of cancer than among those without such a history16.
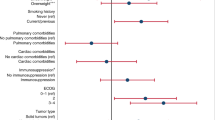
Cancer type, staging and specific therapies are additional risk factors for severe COVID-19 in this patient population. Patients with hematological, lung or breast cancer are more vulnerable than those with other cancers. In the Veneto study, breast and hematological cancers were associated with a higher risk of both hospitalization and death16. Lung cancer was associated with a fourfold risk of death due to SARS-CoV-2 infection16. A study reporting the clinical outcome of COVID-19 in 102 patients with lung cancer showed that the course of SARS-CoV-2 infection in these individuals was longer and more severe than that reported in the general US population21. About one-third of these patients experienced a relatively milder disease course and were treated as outpatients, two-thirds needed hospitalization and one-quarter died. Smoking status and chronic obstructive pulmonary disease (COPD) were the strongest determinants of severity21. 200 patients with COVID-19 and thoracic cancers from eight countries were identified and included in the TERAVOLT registry22. Univariate analyses identified age >65, current or former smoking, presence of any comorbidities and chemotherapy as being associated with increased risk of death. In line with the previous report21, however, multivariate analysis revealed only smoking history as associated with increased risk of death22. In hematological malignancies at older age, diagnosis of acute myeloid leukemia, indolent and aggressive non-Hodgkin lymphoma or plasma cell neoplasms, and severe or critical COVID-19 were associated with worse overall survival23. A retrospective single‐center analysis of 34 patients with hematological malignancies who developed COVID‐19 during follow‐up was conducted in Spain, reporting 11 deaths that could have been predicted by ECOG (Eastern Cooperative Oncology Group) status at disease onset24. Moreover, patients diagnosed with metastatic or stage IV carcinomas may be more susceptible to severe forms of COVID-19 than those with localized neoplasia25. Cancer treatments such as surgery, chemotherapy and immunotherapy have been reported to contribute to the severity of the COVID-19 among patients with cancer19,21,23,25,26,27. For example, a France-based study reported increased risk of deaths from COVID-19 in 178 patients with cancer with age >70, smoking status (current/former), ECOG score ≥2 at last follow-up visit, metastatic disease and use of cytotoxic chemotherapy in the past 3 months19. Separately, two studies reported that immunotherapy within the month before the first symptoms of COVID-19 developed was associated with increased severity and up to 30% death rates in 11 patients27 and 31 patients25, respectively. Age and receipt of immune-checkpoint inhibitor treatment remained significantly associated with COVID severity in a multivariate analysis of 563 patients with cancer25.
Conversely, pediatric patients with cancer seem to be relatively resistant to SARS-CoV-2 infection and severe COVID-19. Among 120 asymptomatic pediatric cancer patients, only 2.5% were positive for SARS-CoV-2, contrasting with a 14.7% rate in their asymptomatic caregivers18,19,28,29. This latter finding favors the possibility that age is a more important risk factor for SARS-CoV-2 infection than cancer.
In conclusion, as of August 2020, it is not clear whether cancer is an independent risk factor for severe COVID-19 or whether the observed cancer-associated risk depends on the peculiar demography and comorbidities of oncology patients30,31. However, given that the time elapsing between cancer diagnosis and SARS-CoV-2 infection was reported to affect the risk of death (in that the shorter the time elapsed, the higher the risk)16, we surmise that the role of therapies and co-medications may so far have been underestimated. Meta-analyses of well-described cancer cohorts, as well as prospective studies, are needed to fully disentangle the role played in COVID-19 severity by carcinogenesis and its clinical management, compared with age, sex and ECOG status.
Common risk factors for severe COVID-19 and cancer
In light of these intriguing data sets, it is important to elucidate the possible cause–effect relationship between severe COVID-19 and pre-existing pro-inflammatory and immunosuppressing conditions related to cancer (Fig. 1) and its treatments (Tables 1 and 2). In this section, we discuss the common risk factors between severe COVID-19 and cancer.
Aging, obesity, metabolic syndrome and exposure to carcinogens are predisposing factors for cancer. Aging, obesity and metabolic syndrome also represent comorbidities that influence susceptibility to and severity of SARS-CoV-2 infection. In patients with cancer, metastatic dissemination and poor ECOG performance status also favor COVID severity. Many genotoxic chemotherapies administered before SARS-CoV-2 viral infection ultimately enhance its severity, likely by inducing immunosuppression and cellular senescence in normal tissues, which in turn maintain local and systemic inflammation, but also through therapy-related adverse events that may include cardiovascular effects, asthenia and propensity to nosocomial infections. Immunosenescence and inflammaging, which are also promoted by aging and obesity, result in declining functions of the innate and adaptive immune systems, exacerbating overt inflammation and cancer dissemination and also increasing vulnerability to SARS-CoV-2 infection and risk of severe COVID-19.
Aging, immunosenescence and inflammaging
Aging increases the incidences of both cancer and SARS-CoV-2 infection32, with potential key commonalities relating to immunosenescence and inflammaging (Fig. 1). Immunosenescence defines a status of declining immune system function associated with, or causing, quantitatively insufficient or qualitatively maladaptive responses to vaccination, infection and neoplasia, as well as an increased incidence of debilitating autoimmune diseases in the elderly population33,34. For example, levels of C-reactive protein are positively associated with senescent CD8+ T cells, plasmablasts and granulocytes in elderly people35. Notably, in patients with COVID-19, lower T cell counts are associated with clinical markers of inflammation, such as ferritin, D dimers and C-reactive protein, whereas high amounts of plasmablasts are associated with disease severity36. A recent immunophenotyping study of young and old individuals diagnosed or not diagnosed with COVID-19 confirmed that COVID-19 promotes age-induced immune cell polarization and gene expression related to inflammation and cellular senescence, and conversely, aging-associated dysregulated immune responses may at least partially account for vulnerability to COVID-19 in the elderly37. Moreover, the generation of naive T cells through thymopoiesis and their priming with novel antigens (such as tumor-specific neoantigens) or infectious agents (such as SARS-CoV-2) are compromised with aging38,39,40. This makes older people more vulnerable to both cancer and viral infections and less able to develop adaptive immune responses during SARS-CoV-2 infection or specific vaccination41,42, unless T cell cross-reactivities against seasonal coronaviruses are also shown to be similarly protective and endowed with reduced risk of antibody-dependent amplified breakthrough infection, compared with SARS-CoV-2-specific immune responses in the elderly43,44. This contrasts with the fact that higher IgG and IgM responses to the SARS-CoV-2 S and N proteins have been observed in elderly patients during the early phase of COVID-19, notwithstanding their higher viral loads than those of younger patients45.
A defect in dendritic cell (DC) fitness with aging has also been reported46. Peripheral germinal center follicular helper T cells are diminished in older people after seasonal influenza vaccination. This post-immunization impairment in the differentiation of follicular helper T cells was recapitulated in 2-year-old mice compared with younger mice, was linked to impaired T cell priming by conventional DCs (cDC2s) and could be restored by topical application of a Toll-like receptor 7 (TLR7) agonist46. Notably, the age-dependent increase in susceptibility to coronaviruses is also associated with an impaired ability of lung DCs to migrate to mediastinal lymph nodes and prime SARS-CoV-specific CD8+ T cells47,48,49. Reduced numbers and impaired functions of DCs and T cells have been reported at the acute phase of severe COVID-19 in 17 patients50. Carcinogenesis is also associated with defective antigen presentation and DC functions, including DC loss and migration defects51,52, which paves the way toward decreased antiviral T cell responses.
In addition to sex and genetics, age is a major factor influencing interindividual differences in transcriptional responses to bacterial, fungal and viral challenges in human peripheral blood mononuclear cells53,54. In the absence of stimulation, the expression of 85% of all genes in mononuclear cells is directly affected by age. After viral stimulation, reduced innate immune responses (for example, type I interferons (IFNs)) are observed in samples from individuals more than 30 years of age. Age (and sex) are also important determinants of humoral immunity, with older individuals (and women) showing higher rates of seropositivity for most antigens53. The reduced ability of aged B cells to increase their metabolism, characterized by a strong reduction in oxidative phosphorylation after activation, contributes to the weakened antibody responses of the elderly to vaccination in general55. A pivotal study analyzing immune responses to the Pandemrix H1N1 influenza vaccine concluded that age was a fundamental component of interindividual variations in the early response (by day 1) to the vaccine, dominated by type I and II IFN fingerprints. Only late (day 7) parameters, such as the rise in transitional plasmablasts usually found in responders, were not influenced by age56.
Separate from such defects in innate and cognate immune responses, ‘inflammaging’ refers to a cytokine dysregulation associated with the age-dependent remodeling of the immune system, as well as to an inability to fine-tune systemic inflammation. Whereas acute, localized inflammation is required for tissue repair responses, systemic and chronic inflammation is harmful. Several common molecular pathways are associated with both aging and low-grade inflammation. For instance, changes in redox equilibrium, defects in the clearance of senescent cells, accumulation of cells with the senescence-associated secretory phenotype (SASP) and reduced autophagy are hallmarks of aging that activate the inflammasome platform, a key orchestrator of cellular inflammatory responses57. Interleukin 6 (IL-6), which has been referred to as the ‘gerontologist’s cytokine’58, is normally present at low levels in the blood, but is increased with aging or frailty (sarcopenia and muscle loss)59 and correlates with mortality60,61. IL-6 is involved in the pathogenesis of many chronic diseases, including cancer62,63. The IL-6–JAK–STAT3 pathway is hyperactivated in many types of cancer, driving the proliferation, survival and invasiveness of tumor cells and suppressing the antitumor immune response. Thus, strategies targeting this pathway have already received US Food and Drug Administration (FDA) approval to treat inflammatory conditions or myeloproliferative neoplasms and to manage certain adverse effects of chimeric antigen receptor–expressing T cells (CAR T cells)64. Given that IL-6 is therapeutically targeted by tocilizumab in the context of COVID-19 to reduce morbidity and mortality related to COVID-19 cytokine release syndrome45, it is conceivable—although it remains to be demonstrated—that inflammaging favors the development of severe SARS-CoV-2 infection. In light of the critical pathophysiological impact of IL-6 and the severity of COVID-19 in individuals with hematological malignancies, prospective controlled studies testing IL-6 receptor (IL-6R) blockade or JAK or STAT3 inhibitors are warranted in this particular subset of patients.
Metabolic syndrome, cancer and COVID-19
Several meta-analyses have revealed an association between type 2 diabetes (T2D) and cancer, with the strongest relationship found for liver and pancreatic cancer, followed by endometrial cancer65. Similarly, morbidly obese individuals (body mass index ≥40 kg/m2) with T2D are more likely to become infected by SARS-CoV-2 and are at a higher risk of complications and death from COVID-1966. Interestingly, individuals with T2D were also at increased risk for SARS and MERS67. Relevant to this, insulin is a key hormonal enhancer of tumor metabolism and growth in obesity-associated insulin resistance68, and treatment of T2D during COVID-19 is being implemented to mitigate disease severity69.
Although human and mouse data analyses revealed that individuals with T2D have reduced ACE2 expression70, patients diagnosed with T2D also have elevated circulating levels of furin, a cellular protease that facilitates viral entry by cleaving the S1 and S2 domain of the SARS-CoV-2 spike protein (S)71. T2D inhibits neutrophil chemotaxis, phagocytosis and intracellular killing of microbes, resulting in impairments in adaptive immunity characterized by an initial delay in the activation of type 1 helper T cell (TH1 cell)–mediated immunity and a late hyperinflammatory response often observed in patients with diabetes72. This could explain the observed links between T2D and increased risk of adverse outcomes for COVID-19 and cancer, both of which depend on protective TH1/cytotoxic T cell type 1 (TH1/Tc1) immune responses.
In addition to facilitating virus entry, the metabolic syndrome may compromise the integrity of the intestinal barrier, a location of SARS-CoV-2 replication. In mice, hyperglycemia increases the permeability of this barrier through glucose transporter 2–dependent transcriptional reprogramming of intestinal epithelial cells and disruption of tight and adherens junctions73. Similarly, in humans, systemic influx of intestinal microbiome products correlates with failing glycemic control73. Given that both SARS-CoV and SARS-CoV-2 can be recovered from feces, infect intestinal epithelial cells and cause diarrhea74,75, hyperglycemia-induced dysfunction of the intestinal barrier might facilitate bacterial translocation, thus favoring systemic inflammation and immunosuppression. Interestingly, in a humanized mouse model of MERS-CoV infection on a high-fat diet, the course of infection was more severe and prolonged in male mice and was characterized by IL-17-producing helper T cell (TH17 cell) responses67, which are known to be pro-angiogenic and immunosuppressive in the course of cancer progression76. Notably, obese individuals contract more bacterial, viral and fungal infections than do lean counterparts, and respond relatively poorly to vaccination against influenza, hepatitis B, tetanus and rabies77. Obesity, alone or together with metabolic syndrome, induces defects in B cells similar to those associated with aging, contributing to systemic and B cell–intrinsic inflammation as well as to a surge in autoantibodies77.
Immunosuppression, lymphopenia, neutrophilia and interferon deficiency
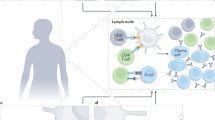
Through their participation in immunosurveillance, lymphocytes control the incidence, progression and therapeutic response of cancers78. CD4+ and CD8+ T lymphocytes recognize tumor cells expressing immunodominant epitopes presented by major histocompatibility complex class II and class I, respectively. CD4+ lymphopenia, a hallmark of immunosuppressive viral infection, occurs in ~20% of patients with advanced pancreatic cancer, melanoma, non-Hodgkin’s lymphoma, breast cancer, sarcomas or hepatocellular carcinoma but is rare (~2%) in patients with localized disease79,80,81,82. In fact, cancer-associated lymphopenia, mostly affecting CD4+ T cell counts, has been reported to increase the risk of comorbidities (febrile neutropenia), resistance to a range of therapies and mortality across many cancer types79. Lymphopenia often accompanies cancer diagnosis, treatment or progression and is a side effect of chemotherapy and steroids. Radiotherapy also negatively impacts circulating lymphocyte counts83. An increased number of circulating neutrophils is often combined with decreased lymphocyte counts, resulting in a marked elevation of the neutrophil-to-lymphocyte ratio84. A high neutrophil-to-lymphocyte ratio is a poor prognostic marker and predicts short cancer-specific progression-free survival after blockade of programmed cell death protein 1 (PD-1)84, as well as severe COVID-1985. Beyond lymphopenia, a reduction in T cell receptor diversity and a functional impairment of other lymphoid and myeloid immune cells (such as natural killer (NK) cells, monocytes, DCs, and memory CD4+ and CD8+ T cells) have been detected in patients with localized primary tumors such as breast cancer, colon carcinoma and hepatocellular carcinoma79,86,87,88.
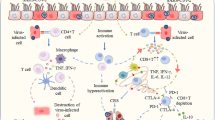
Given the critical role of T effector lymphocytes in eliminating virus-infected cells, an attenuated and functionally compromised T cell pool may pave the way toward the higher incidence and severity of COVID-19 in patients with cancer. Indeed, the outcome of COVID-19 may be determined by a ‘race’ between the cellular immune system that mounts a response to eliminate virus-infected cells and the immunosuppressive action of the pathogen89 (Fig. 2). Individuals who present with detectable memory B and T cell responses against seasonal coronaviruses may be able to mobilize a pool of effector T cells and mount neutralizing antibody responses that may, at least in part, prevent the thrombotic microangiopathy associated with SARS-CoV-2 viral endothelial infection90,91,92. Moreover, MERS-CoV and SARS-CoV-2 trigger apoptosis and necroptosis of T cells and reduce lymphopoietin IL-7 as an immunosuppressive strategy93.
Cancer, at an advanced or metastatic stage, may compromise the delicate equilibrium between viral replication and appropriate innate (for example, type I and III IFN) and cognate immune responses (for example, memory TH1 responses and antibody-secreting cell cross-reactivity with other beta coronaviruses) in the lung alveolar epithelium and mediastinal lymph nodes. The SARS-CoV-2 virus infects endothelial cells of the lung alveolar capillaries, inducing microthrombi and severe endothelial injury. The failure of the immune system to control early viral replication and to prevent endothelial injury may lead to a marked release of chemokines, cytokines and/or alarmins and a viral sepsis initiated or maintained by pulmonary or medullary hematopoiesis. Gr, granulocyte; HCoV, human coronavirus; IFN, interferon; IL, interleukin; ISG, interferon-stimulating gene; ORF, open reading frame; RGD, Arg‐Gly‐Asp.
If people with cancer start this ‘race’ with a handicap due to pre-existing T cell defects, this makes them particularly susceptible to COVID-19-associated severe pneumonia or systemic organ failure93. Conversely, it is unclear whether cancer-associated immunosuppressive cells—such as regulatory T cells, TH17 cells, myeloid-derived suppressor cells and ‘exhausted’ PD-1 ligand–positive T cells—might mitigate lung inflammation caused by SARS-CoV-279,94,95,96,97. Longitudinal high-dimensional immunomonitoring will help delineate favorable and deleterious cancer-associated immune factors for each stage of COVID-19 infection.
Type I and II IFN responses are intertwined and essential for long-term protective anticancer and antiviral immune responses98,99,100. Defective type I IFN responses by conventional or plasmacytoid DCs during natural immunosurveillance, or by tumor cells after chemotherapy or radiotherapy, are associated with tumor progression101,102,103,104. Intestinal dysbiosis, as well as tumor-intrinsic genetic defects, account for aberrant IFN-α/β receptor (IFNAR) signaling, preventing full efficacy of immunotherapy in patients with tumors101. RNA viruses engage pattern-recognition receptors (e.g., TLR3, TLR7 and TLR8, RIG-I, MDA5) on antigen-presenting cells, culminating in the induction of type I IFNs and an endoplasmic reticulum stress response that shuts down viral protein translation while igniting innate effector cells, such as NK cells or DCs105,106. Elegant studies have revealed the beneficial role of type I IFN signaling at early steps of infection and the protective impact of type II IFNs released by airway memory CD4+ T cells, or intranasal instillations of recombinant IFNγ, against SARS-CoV infection in mice107. Separately, treatment with the TLR3 agonist poly(I:C) protected mice against highly pathogenic coronavirus species, including group 2c (MERS-like) coronaviruses108,109. Similarly, TLR signaling through the TRIF adaptor protein protected mice from lethal SARS-CoV disease110. However, in stark contrast to other beta coronaviruses, SARS-CoV-2 fails to induce—or perhaps subverts—a type I IFN response in immune cells111. Thus, in response to SARS-CoV-2, the host does not increase the production of type I and type III IFNs but instead produces high levels of inflammatory chemokines and cytokines, particularly IL-6, thus stimulating emergency hematopoiesis and attracting granulocytes and monocytes to lung lesions111,112,113 (Fig. 2). As a failing immune response enables sustained viral replication, a positive feedback loop becomes established, eventually allowing the virus to prevail. These findings have been substantiated by in vitro studies in which normal lung parenchyma from five healthy human donors was infected with SARS-CoV versus SARS-CoV-2. In contrast to SARS-CoV, SARS-CoV-2 was highly replicative and poorly immunogenic, failing to trigger a type I IFN response and TH1 chemokine release114.
However, the timing of activation of the type I IFN pathway is key for antiviral and antitumor immune responses. For example, in separate mouse-based studies, delayed IFNβ treatment failed to effectively inhibit virus replication of SARS-CoV-2115 and MERS-CoV116, increased infiltration and activation of monocytes, macrophages and neutrophils in the lungs115,116 and enhanced the expression of pro-inflammatory cytokines, resulting in fatal pneumonia during SARS-CoV infection116,117 and in tumor resistance to PD-1 blockade115,116,117,118.
Altogether, these results imply that cancer and COVID-19 may be concomitantly aggravated by comorbidities such as aging, metabolic disorders, and innate and cognate immunosuppression (Fig. 1). These comorbidities may also compromise the efficacy of immune-based anticancer and antiviral therapies.
Cancer therapeutics with possible pro-COVID-19 effects
Tumor control achieved by oncological treatments is counterbalanced by the cardiovascular toxicities of cytotoxic agents, which often cause premature discontinuation of an effective therapy or undermine overall survival. An association between cancer and cardiovascular diseases, as well as a direct relationship between hypertension and cancer incidence and mortality, have been documented119. Thus, arterial hypertension (AHT) is both the most common comorbidity of cancer and a frequent adverse effect of antineoplastic therapies120. Pre-existing AHT is known to increase the risk of other cardiac adverse events due to oncologic treatments, in particular heart failure121. Many antineoplastic treatments (of which a non-exhaustive list is provided in Tables 1 and 2), particularly small molecules or antibodies targeting the growth factor VEGF or its receptor VEGF-R2 and tyrosine kinase inhibitors, cause AHT, compromising the long-term outcome of chemotherapy120. All these side effects theoretically complicate the prognosis of COVID-1925. However, and notwithstanding the high serum VEGF levels found in patients suffering from severe SARS-CoV-2, clinical trials in China are currently assessing the effect of targeting VEGF with bevacizumab in COVID-19 (NCT04275414) in preventing immunothrombosis.
Cytotoxic drugs administered at ablative or non-myeloablative dosages stimulate bone marrow progenitors and the exodus of both immature and mature cells, including granulocytes, monocytes and platelets. As previously reported, the resulting leukocytosis and production of the cytokine G-CSF could facilitate the differentiation of myeloid-derived suppressor cells and modulate neutrophil-to-lymphocyte ratios, paving the way to tumor progression, metastasis and poor clinical outcome122.
Genotoxic chemotherapies induce cellular senescence in normal tissues, where they promote local and systemic inflammation that causes or exacerbates the debilitating effects of chemotherapy15. In mice, ablating senescent cells reduces many side effects of cytotoxic agents, including cancer recurrence, cardiac dysfunction and myelosuppression15. Moreover, the risk of chemotherapy-induced asthenia is higher in people with increased expression of a senescence marker in T cells before chemotherapy15. Accordingly, after and during chemotherapy, patients with cancer are particularly susceptible to severe COVID-1919.
Radiation-induced pulmonary fibrosis (RIPF) is a common complication of thoracic radiotherapy for lung and breast cancer, observed in 16–28% of patients123. RIPF leads to irreversible destruction of lung architecture and disruption of gas exchange. Because the pathophysiology of RIPF features epithelial cell dysfunction and senescence, pro-inflammatory cytokine release and dysfunction of innate and adaptive immunity, it is not surprising that patients with lung cancer may have an elevated susceptibility to severe COVID-1921,124. Nevertheless, with appropriate dosing and timing, radiotherapy may be beneficial against acute respiratory distress syndrome, with single-fraction radiation or short courses of radiation being recommended125,126.
An association of checkpoint inhibitor–based immunotherapy with the aggravation of COVID-19, including increased hospitalization and severe respiratory conditions, was first reported in 31 patients25. This negative prognostic link was independent of age, cancer type and other comorbid conditions or coadministered medications such as steroids25. In this case, immune-checkpoint inhibitors may have exacerbated immune-related pneumonitis or T cell cytokine release, as previously discussed127. Given that many therapeutic actions currently used in oncology may increase the risk of severe SARS-CoV-2 infection, current guidelines related to cancer care during the COVID-19 crisis advise the postponement of all non-mandatory cancer therapies128.
In recent years we have accumulated an unprecedented understanding of the molecular pathways and immune-tolerance mechanisms governing the incidence and severity of human neoplasia, leading to a large swath of targeted anticancer therapies and immunotherapies. Despite their specificity, however, small-molecule inhibitors and antibody-based therapies induce both on- and off-target effects—the latter including immune-related pneumonitis and diabetes, among other conditions—that could increase the susceptibility of patients with cancer to COVID-19 (Fig. 3, Tables 1 and 2).
Major side effects triggered by the main classes of compounds in the oncological armamentarium, including conventional therapies (such as cytotoxic chemotherapy, hormone therapy and radiotherapy), targeted therapies (such as TKI and mTOR inhibitors) and immunotherapies (such as immune-checkpoint inhibitors and CAR T cells), that can exacerbate COVID-19. On- or off-target unwanted effects of the drugs listed in each rectangle are indicated in uppercase letters. See also Tables 1 and 2. BiTE, bispecific T cell engagers; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; LH-RH, luteinizing hormone–releasing hormone; mTOR, mammalian target of rapamycin; TKI, tyrosine kinase inhibitor.
Cancer therapeutics with potential anti-COVID-19 effects
The quest for safe agents capable of inhibiting SARS-CoV-2 infection and replication has been intense over the past several months, spurring screening campaigns aimed at drug repurposing129. We have identified a number of antiviral drugs with potential antineoplastic properties and, reciprocally, anticancer agents with potential antiviral effects (Tables 1 and 2). In this section we discuss some of these therapeutic agents.
Interferon-based therapies
Recombinant IFNγ and IFNα2b have been widely used against cancer, alone or combined with other cancer treatment modalities130. Because SARS-CoV-2 compromises the paradigmatic type 1 interferon antiviral response111,113,114, IFN administration—either locally by vapor inhalation or systemically, and alone or in combination with ribavirin106,131, lopinavir/ritonavir, remdesivir132 or hydroxychloroquine—has arisen as a promising treatment approach against COVID-19131 (Tables 1 and 2). IFNγ as an inhaled aerosol has been found to be effective against tuberculosis in a controlled clinical trial and to be safe and ameliorate pulmonary function in a phase 1 clinical trial of patients with idiopathic pulmonary fibrosis133. Thus, inhaled IFNγ has been proposed as a treatment against COPD, tuberculosis and idiopathic pulmonary fibrosis133. It will be interesting to follow SARS-CoV-2 viral loads, virulence and the expression of type I IFN–related genes in the blood and lungs of patients with cancer over the course of their cytotoxic therapies, to estimate the suitability of IFN supplementation in treating COVID-19. Alternatively, another way to increase the systemic circulating levels of type I IFNs would be to use pattern-recognition-receptor agonists. For instance, subcutaneous administrations of a TLR9 agonist increases circulating levels of type I IFNs and decreases hepatitis C viral loads107.
Immune-checkpoint blockers
On theoretical grounds, immunotherapy with immune-checkpoint blockers might simultaneously boost cytotoxic T lymphocyte (CTL) immune responses134 against virus-infected and neoplastic cells. At this stage, however, there is little information on the role of T cells during the severe phase of COVID-19. Indeed, T cells also might cause lung immunopathology, meaning that their therapeutic reactivation would exacerbate the disease135. In contrast, it is also possible that CTL exhaustion accompanying respiratory virus–induced pneumonitis might render such cells unable to eliminate inflammatory myeloid cells, in which case immune-checkpoint blockers might have anti-inflammatory effects. Indeed, virus-induced secondary hemophagocytic lymphohistiofibrosis (HLH) has been reported to result from inappropriate and prolonged macrophages activation10,136. Supporting the view that T cell exhaustion might be causal in the HLH syndrome, a recent clinical study showed a remarkable efficacy of anti-PD-1 antibodies in treating HLH induced by Epstein–Barr virus in four out of six patients treated137. However, although a retrospective analysis of 62 patients with lung cancer adjusted for gender and smoking status showed the non-inferiority of anti-PD-1 treatment in cases of concomitant COVID-19 and non-small-cell lung cancer138, the contrary effect has been reported recently across various malignancies, as discussed above25.
IL-6–JAK–STAT3 blockade
As outlined above, overt inflammation accompanied by elevated plasma IL-6 levels is associated with severe COVID-1910 and disseminated malignancies64. Despite the absence of a causative link between the manifestation of a cytokine release syndrome and COVID-19 severity, investigators have attempted to interfere with this inflammatory cascade. Tocilizumab, an anti–human IL-6R monoclonal antibody, was recently approved to treat severe SARS-CoV-2-related pulmonary complications by the National Health Commission of the People’s Republic of China. Observational studies conducted in China in patients with severe COVID-19 receiving tocilizumab showed an improvement of clinical and radiological outcome139 and attenuation of the hyperactivated inflammatory immune responses140 as well as restoration of a robust T cell-associated adaptive immunity141 in these patients142,143. Meta-analyses of studies of IL-6R blockade to treat rheumatoid arthritis revealed a drastic reduction of neutrophil counts in chronically treated patients144. Such neutrophil depletion might alleviate lung inflammation in severe COVID-19, as emergency hematopoiesis and bone marrow exodus of immature neutrophils and pre-neutrophils may predict the switch between moderate and severe cases112. However, given that the first randomized trial assessing the efficacy of IL-6R blockade in overt pneumonia (the COVACTA phase 3 trial, NCT04320615) did not reach its primary endpoint145, stratification of patients may be necessary to reach clinical significance.
Androgen-deprivation therapy
Androgen-receptor signaling enhances TMPRSS2 expression in non-prostatic tissues, including lungs146, potentially contributing to the increased vulnerability of male individuals to SARS-CoV-2. Androgen-deprivation therapy (ADT) decreases the levels of TMPRSS2 and may be used to counter the severity of SARS-CoV-2 infection in male patients, potentially in combination with other inhibitors of viral entry or replication. Accordingly, in one study, patients with prostate cancer receiving ADT had a significantly lower risk of severe COVID-19 than patients who did not receive ADT147. An even greater difference was found when comparing patients with prostate cancer receiving ADT to patients with other types of malignancies147. Hence, ADT with luteinizing hormone–releasing hormone (LH-RH) modulators or AR inhibitors (Tables 1 and 2) may be worth evaluating as interceptive medications against COVID-19 in prophylactic therapy148, as well as therapeutic measures for high-risk male patients147.
Other small molecules
Based on its capacity to inhibit autophagy, hydroxychloroquine (HCQ) is being evaluated in clinical trials to treat autophagy-dependent cancers149. In spite of initial reports that HCQ might reduce the duration of SARS-CoV-2 infection and the severity of COVID-19150, later reports failed to confirm significant effects of the drug151,152, suggesting that the simultaneous treatment of cancer and COVID-19 with HCQ is not advisable. Nonetheless, there may be other small molecules with dual anticancer and anti-SARS-CoV-2 properties. For example, inhibitors of translation elongation factors may have such a dual activity because both cancer cells and coronaviruses rely on cap-dependent mRNA translation. A recent study that screened a large library of compounds for their capacity to inhibit the replication of SARS-CoV-2 identified inhibitors of elongation factor 1A (eEF1A) and eukaryotic initiation factor 4A (eIF4A)153 that are currently being evaluated to treat multiple myeloma and KRAS-mutated cancers, respectively153,154(Tables 1 and 2). A separate report screened a library of FDA-approved drugs for inhibitors of coronavirus replication and identified Abelson (ABL) kinase inhibitors, including the anticancer drug imatinib mesylate, as inhibitors of both SARS-CoV and MERS-CoV in vitro155, and a later study confirmed that imatinib mesylate inhibits SARS-CoV-2 replication in cultured cells as well156. Although no in vivo data are yet available, the use of imatinib mesylate to treat COVID-19 is an active area of research (Tables 1 and 2). It should be noted that imatinib mesylate has the capacity to stimulate T and NK lymphocyte–mediated anticancer responses, suggesting that it has immunostimulatory effects157. Whether it can also stimulate immune responses against SARS-CoV-2 remains to be investigated. Other small molecules (including JAK1/2 inhibitors158,159 and anti-CD26 antibody160,161,162; see Tables 1 and 2) currently in use for hematological malignancies to alleviate graft-versus-host disease, cytokine storms or overt inflammatory responses may also be of interest against severe COVID-19, as discussed elsewhere159,163. Many additional antiviral and tumoricidal therapeutics or interceptive strategies (such as the anti-inflammatory use of low-dose thoracic irradiation164,165,166,167,168,169,170,171 or prophylactic vitamin D172,173,174,175,176,177,178,179,180) listed in Tables 1 and 2 are currently under investigation.
Conclusions and future directions
The current COVID-19 crisis has a lesser impact on healthy and fit children and adolescents, while claiming its deadly toll among all other segments of the population: the sick, the unfit and the elderly, including patients with cancer. Malignant disease predisposes to severe COVID-19 for multiple reasons, primarily because (i) patients with cancer fall into general at-risk categories because of their average advanced age, predisposing factors such as obesity and smoking, and comorbidities such as T2D and hypertension; (ii) cancer intrinsically has negative effects on patients’ general health status; and (iii) antineoplastic therapies such as surgery, chemotherapy and radiotherapy may debilitate the immune system and cause immunosenescence and inflammaging. However, whether cancer per se is an independent risk factor for severe COVID-19 remains to be elucidated. It should also be kept in mind that during the COVID-19 outbreak, morbidity and mortality of patients with cancer may have been substantially affected not only by the viral disease itself but also by the extreme pressure exerted by COVID-19 on the healthcare system, which led to the postponement of cancer treatments and the allocation of scarce resources, such as intensive care beds and ventilators, to patients with better prognoses181,182,183. During the present COVID-19 pandemic, oncology departments are frequently confronted with the challenge of treating patients with both cancer and COVID-19, raising a strong argument for exploring therapeutic strategies that could simultaneously improve both diseases. Several drugs that have direct inhibitory effects on SARS-CoV-2 replication in vitro184,185,186,187,188 (and that still require further characterization in clinical trials; Table 3) are also known for their potential anticancer effects, supporting the idea that such agents, including imatinib mesylate and inhibitors of cap-dependent translation, might have a dual therapeutic activity against cancer and COVID-19. Given the uncertainties about the benefits of PD-1 and/or PD-L1 or IL-6R blockade25,145, other possibilities are being investigated, such as passive transfer of neutralizing anti-SARS-CoV-2 antibodies for frail patients at a moderate to severe stage of COVID-19189. Finally, active vaccination will be an option for patients at high risk of developing severe COVID-19 but still capable of mounting protective antiviral T cell responses41. However, candidate vaccines will have to undergo large-scale phase 3 clinical trials to assess their effectiveness and safety, so the earliest regulatory approvals and roll-outs are not expected before late 2020 or early 2021. All these possibilities await urgent investigation to allow clinical oncologists to navigate between cancer and COVID-19 in full compliance with the Hippocratic Oath: primum non nocere—first, do no harm.
Change history
08 February 2022
A Correction to this paper has been published: https://doi.org/10.1038/s43018-022-00342-9
References
World Health Organization. Coronavirus Disease (COVID-19) Situation Reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed 15 August 2020).
Worldometer. Coronavirus Update (Live): 24,643,164 cases and 835,843 deaths from COVID-19 virus pandemic. Worldometer. https://www.worldometers.info/coronavirus/ (accessed 15 August 2020).
Li, W. et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454 (2003).
Choi, S.-Y., Bertram, S., Glowacka, I., Park, Y. W. & Pöhlmann, S. Type II transmembrane serine proteases in cancer and viral infections. Trends Mol. Med. 15, 303–312 (2009).
Lucas, J. M. et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 4, 1310–1325 (2014).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8 (2020).
Sungnak, W. et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 26, 681–687 (2020).
Hamming, I. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203, 631–637 (2004).
Wu, C. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 180, 1–11 (2020).
Mehta, P. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034 (2020).
Tan, L. et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target. Ther. 5, 33 (2020).
Ma, J., Lew, L. & Jeong-ho, L. A third of coronavirus cases may be ‘silent carriers’, classified Chinese data suggests. South China Morning Post https://www.scmp.com/news/china/society/article/3076323/third-coronavirus-cases-may-be-silent-carriers-classified
Kamboj, M. & Sepkowitz, K. A. Nosocomial infections in patients with cancer. Lancet Oncol. 10, 589–597 (2009).
Ray-Coquard, I. et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 69, 5383–5391 (2009).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Disc. 7, 165–176 (2017).
Rugge, M., Zorzi, M. & Guzzinati, S. SARS-CoV-2 infection in the Italian Veneto region: adverse outcomes in patients with cancer. Nat. Cancer 1, 784–788 (2020).
Liang, W. et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 21, 335–337 (2020).
Yu, J., Ouyang, W., Chua, M. L. K. & Xie, C. SARS-CoV-2 transmission in cancer patients of a tertiary hospital in Wuhan. JAMA Oncol. 6, 1108–1110 (2020).
Albiges, L. et al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the Gustave Roussy cohort. Nat. Cancer https://doi.org/10.1038/s43018-020-00120-5 (2020).
Assaad, S. et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur. J. Cancer 135, 251–259 (2020).
Luo, J. et al. COVID-19 in patients with lung cancer. Ann. Oncol. https://doi.org/10.1016/j.annonc.2020.06.007 (2020).
Garassino, M. C. et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 21, 914–922 (2020).
Passamonti, F. et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. https://doi.org/10.1016/S2352-3026(20)30251-9 (2020).
Martín-Moro, F. et al. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br. J. Haematol. 190, e16–e20 (2020).
Robilotti, E. V. et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 26, 1218–1223 (2020).
Dai, M. et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 10, 783–791 (2020).
Wu, Q. et al. Clinical outcomes of coronavirus disease 2019 (COVID-19) in cancer patients with prior exposure to immune checkpoint inhibitors. Cancer Commun. 40, 374–379 (2020).
Choi, S.-H., Kim, H. W., Kang, J.-M., Kim, D. H. & Cho, E. Y. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin. Exp. Pediatr. 63, 125–132 (2020).
Boulad, F., Kamboj, M., Bouvier, N., Mauguen, A. & Kung, A. L. COVID-19 in children with cancer in New York City. JAMA Oncol. 6, 1459–1460 (2020).
Kuderer, N. M. et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 395, 1907–1918 (2020).
Lee, L. Y. et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 395, 1919–1926 (2020).
Wang, D. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J. Am. Med. Assoc. 323, 1061–1069 (2020).
Stahl, E. C. & Brown, B. N. Cell therapy strategies to combat immunosenescence. Organogenesis 11, 159–172 (2015).
Pawelec, G. Age and immunity: what is ‘immunosenescence’? Exp. Gerontol. 105, 4–9 (2018).
Stevenson, A. J. et al. Trajectories of inflammatory biomarkers over the eighth decade and their associations with immune cell profiles and epigenetic ageing. Clin. Epigenetics 10, 159 (2018).
Mathew, D. et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 369, eabc8511 (2020).
Zheng, Y. et al. A human circulating immune cell landscape in aging and COVID-19. Protein Cell https://doi.org/10.1007/s13238-020-00762-2 (2020).
Donnelly, C. A. et al. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 361, 1761–1766 (2003).
Zhang, Y. & Ertl, H. C. Aging: T cell metabolism within tumors. Aging 8, 1163–1164 (2016).
Clave, E. et al. Human thymopoiesis is influenced by a common genetic variant within the TCRA–TCRD locus. Sci. Transl. Med. 10, eaao2966 (2018).
Yu, J. et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 369, 806–811 (2020).
Deng, X. et al. Genomic surveillance reveals multiple introductions of SARS-CoV-2 into Northern California. Science 369, 582–587 (2020).
Mateus, J. et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science https://doi.org/10.1126/science.abd3871 (2020).
Halstead, S. B. & Katzelnick, L. COVID 19 vaccines: should we fear ADE? J. Infect. Dis. https://doi.org/10.1093/infdis/jiaa518 (2020).
Wu, J. et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Preprint at medRxiv https://doi.org/10.1101/2020.07.21.20159178 (2020).
Stebegg, M. et al. Rejuvenating conventional dendritic cells and T follicular helper cell formation after vaccination. eLife 9, e52473 (2020).
Zhao, J., Zhao, J., Legge, K. & Perlman, S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J. Clin. Invest. 121, 4921–4930 (2011).
Murasko, D. M. & Jiang, J. Response of aged mice to primary virus infections. Immunol. Rev. 205, 285–296 (2005).
Roberts, A. et al. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 3, e5 (2007).
Zhou, R. et al. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity https://doi.org/10.1016/j.immuni.2020.07.026 (2020).
Theisen, D. J. et al. Batf3-dependent genes control tumor rejection induced by dendritic cells independently of cross-presentation. Cancer Immunol. Res. 7, 29–39 (2019).
Palucka, K., Ueno, H., Fay, J. & Banchereau, J. Dendritic cells and immunity against cancer. J. Intern. Med. 269, 64–73 (2011).
Scepanovic, P. et al. Human genetic variants and age are the strongest predictors of humoral immune responses to common pathogens and vaccines. Genome Med. 10, 59 (2018).
Piasecka, B. et al. Distinctive roles of age, sex, and genetics in shaping transcriptional variation of human immune responses to microbial challenges. Proc. Natl Acad. Sci. USA 115, E488–E497 (2018).
Kurupati, R. K., Haut, L. H., Schmader, K. E. & Ertl, H. C. Age-related changes in B cell metabolism. Aging 11, 4367–4381 (2019).
Sobolev, O. et al. Adjuvanted influenza-H1N1 vaccination reveals lymphoid signatures of age-dependent early responses and of clinical adverse events. Nat. Immunol. 17, 204–213 (2016).
Cuervo, A. M. & Macian, F. Autophagy and the immune function in aging. Curr. Opin. Immunol. 29, 97–104 (2014).
Ershler, W. B. & Keller, E. T. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 51, 245–270 (2000).
Nelke, C., Dziewas, R., Minnerup, J., Meuth, S. G. & Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 49, 381–388 (2019).
Puzianowska-Kuźnicka, M. et al. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun. Ageing 13, 21 (2016).
Varadhan, R. et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 69, 165–173 (2014).
Weiss, T. W., Arnesen, H. & Seljeflot, I. Components of the interleukin-6 transsignalling system are associated with the metabolic syndrome, endothelial dysfunction and arterial stiffness. Metabolism 62, 1008–1013 (2013).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. Gerontol. A Biol. Sci. Med. Sci. 69, S4–9 (2014).
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Oberaigner, W. et al. Increased cancer incidence risk in type 2 diabetes mellitus: results from a cohort study in Tyrol/Austria. BMC Public Health 14, 1058 (2014).
Muniyappa, R. & Gubbi, S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 318, E736–E741 (2020).
Kulcsar, K. A., Coleman, C. M., Beck, S. E. & Frieman, M. B. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight 4, e131774 (2019).
Perry, R. J. & Shulman, G. I. Mechanistic links between obesity, insulin, and cancer. Trends Cancer 6, 75–78 (2020).
Longo, M. et al. Treating type 2 diabetes in COVID-19 patients: the potential benefits of injective therapies. Cardiovasc. Diabetol. 19, 115 (2020).
Chen, J. et al. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 19, e13168 (2020).
Coutard, B. et al. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 176, 104742 (2020).
Hodgson, K. et al. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 144, 171–185 (2015).
Thaiss, C. A. et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359, 1376–1383 (2018).
Reisinger, E. C., Fritzsche, C., Krause, R. & Krejs, G. J. Diarrhea caused by primarily non-gastrointestinal infections. Nat. Clin. Pract. Gastroenterol. Hepatol. 2, 216–222 (2005).
Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54 (2020).
Martin, F., Apetoh, L. & Ghiringhelli, F. Controversies on the role of Th17 in cancer: a TGF-β-dependent immunosuppressive activity? Trends Mol. Med. 18, 742–749 (2012).
Frasca, D. & Blomberg, B. B. The impact of obesity and metabolic syndrome on vaccination success. Interdiscip. Top. Gerontol. Geriatr. 43, 86–97 (2020).
Galluzzi, L., Buqué, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Ménétrier-Caux, C., Ray-Coquard, I., Blay, J.-Y. & Caux, C. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J. Immunother. Cancer 7, 85 (2019).
Bedimo, R. J., McGinnis, K. A., Dunlap, M., Rodriguez-Barradas, M. C. & Justice, A. C. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J. Acquir. Immune Defic. Syndr. 52, 203–208 (2009).
Péron, J. et al. CD4 lymphopenia to identify end-of-life metastatic cancer patients. Eur. J. Cancer 49, 1080–1089 (2013).
Trédan, O. et al. Patients with metastatic breast cancer leading to CD4+ T cell lymphopaenia have poor outcome. Eur. J. Cancer 49, 1673–1682 (2013).
Meyer, K. K. Radiation-induced lymphocyte-immune deficiency. A factor in the increased visceral metastases and decreased hormonal responsiveness of breast cancer. Arch. Surg. 101, 114–121 (1970).
Ocana, A., Nieto-Jiménez, C., Pandiella, A. & Templeton, A. J. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol. Cancer 16, 137 (2017).
Zhang, B. et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front. Mol. Biosci. 7, 157 (2020).
Rocca, Y. S. et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun. 19, 76–85 (2013).
Verronèse, E. et al. Immune cell dysfunctions in breast cancer patients detected through whole blood multi-parametric flow cytometry assay. Oncoimmunology 5, e1100791 (2015).
Chittezhath, M. et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity 41, 815–829 (2014).
Chen, Y. & Li, L. SARS-CoV-2: virus dynamics and host response. Lancet Infect. Dis. 20, 515–516 (2020).
Braun, J. et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature https://doi.org/10.1038/s41586-020-2598-9 (2020).
Grifoni, A. et al. A Sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe 27, 671–680.e2 (2020).
Ng, K. W. Pre-existing and de novo humoral immunity to SARS-CoV-2 in humans. Preprint at bioRxiv https://doi.org/10.1101/2020.05.14.095414 (2020).
Chu, H. et al. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 213, 904–914 (2016).
Jacquelot, N. et al. Immunophenotyping of stage III melanoma reveals parameters associated with patient prognosis. J. Invest. Dermatol. 136, 994–1001 (2016).
An, Y. et al. Transforming growth factor-β and peripheral regulatory cells are negatively correlated with the overall survival of hepatocellular carcinoma. World J. Gastroenterol. 24, 2733–2740 (2018).
Feng, P. et al. The alteration and clinical significance of Th1/Th2/Th17/Treg cells in patients with multiple myeloma. Inflammation 38, 705–709 (2015).
Shen, P., Wang, A., He, M., Wang, Q. & Zheng, S. Increased circulating Lin–/low CD33+ HLA-DR– myeloid-derived suppressor cells in hepatocellular carcinoma patients. Hepatol. Res. 44, 639–650 (2014).
Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414 (2015).
Musella, M., Manic, G., De Maria, R., Vitale, I. & Sistigu, A. Type-I-interferons in infection and cancer: unanticipated dynamics with therapeutic implications. Oncoimmunology 6, e1314424 (2017).
Lee, A. J. & Ashkar, A. A. The dual nature of type I and type II interferons. Front. Immunol. 9, 2061 (2018).
Fuertes, M. B. et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J. Exp. Med. 208, 2005–2016 (2011).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Sistigu, A. et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 20, 1301–1309 (2014).
Formenti, S. C. et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 24, 1845–1851 (2018).
Xue, M. et al. The PERK arm of the unfolded protein response negatively regulates transmissible gastroenteritis virus replication by suppressing protein translation and promoting type I interferon production. J. Virol. 92, e00431–18 (2018).
Sallard, E., Lescure, F.-X., Yazdanpanah, Y., Mentre, F. & Peiffer-Smadja, N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 178, 104791 (2020).
Zhao, J. et al. Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity 44, 1379–1391 (2016).
Zhao, J. et al. Intranasal treatment with poly(I•C) protects aged mice from lethal respiratory virus infections. J. Virol. 86, 11416–11424 (2012).
Kumaki, Y., Salazar, A. M., Wandersee, M. K. & Barnard, D. L. Prophylactic and therapeutic intranasal administration with an immunomodulator, Hiltonol® (Poly IC:LC), in a lethal SARS-CoV-infected BALB/c mouse model. Antiviral Res. 139, 1–12 (2017). doi:.
Totura, A. L. et al. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio 6, e00638–15 (2015).
Blanco-Melo, D. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell https://doi.org/10.1016/j.cell.2020.04.026 (2020).
Silvin, A. et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell https://doi.org/10.1016/j.cell.2020.08.002 (2020).
Hadjadj, H. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724 (2020).
Chu, H. et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciaa410 (2020).
Israelow, I. et al. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J.Exp. Med. 217, e20201241 (2020).
Channappanavar, R. et al. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Invest. 129, 3625–3639 (2019).
Channappanavar, R. et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 19, 181–193 (2016).
Jacquelot, N. et al. Sustained type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res. 29, 846–861 (2019).
Bravo-Jaimes, K. et al. Opportunities for improved cardiovascular disease prevention in oncology patients. Curr. Opin. Cardiol. 35, 531–537 (2020).
Giacomo, T. et al. Arterial hypertension in cancer: the elephant in the room. Int. J. Cardiol. 281, 133–139 (2019).
Essa, H., Pettitt, A. R. & Lip, G. Y. H. Hypertension and cardiovascular risk factors when treating cancer patients: underrecognised and undertreated. J. Hum. Hypertens. https://doi.org/10.1038/s41371-020-00400-8 (2020).
Tavakkoli, M., Wilkins, C. R., Mones, J. V. & Mauro, M. J. A novel paradigm between leukocytosis, G-CSF secretion, neutrophil-to-lymphocyte ratio, myeloid-derived suppressor cells, and prognosis in non-small cell lung cancer. Front. Oncol. 9, 295 (2019).
Jin, H. et al. Radiation-induced lung fibrosis: preclinical animal models and therapeutic strategies. Cancers 12, 1561 (2020).
Addeo, A., Obeid, M. & Friedlaender, A. COVID-19 and lung cancer: risks, mechanisms and treatment interactions. J. Immunother. Cancer 8, e000892 (2020).
Cosset, J. M., Deutsch, É., Bazire, L., Mazeron, J.-J. & Chargari, C. [Low dose lung radiotherapy for COVID-19-related cytokine storm syndrome: Why not?]. Cancer Radiother. 24, 179–181 (2020).
Zhao, Z., Yang, C. & Li, C. Strategies for patient with cancer during COVID-19 pandemic. Asia Pac. J. Clin. Oncol. https://doi.org/10.1111/ajco.13363 (2020).
Vardhana, S. A. & Wolchok, J. D. The many faces of the anti-COVID immune response. J. Exp. Med. 217, e20200678 (2020).
Routy, B., Derosa, L., Zitvogel, L. & Kroemer, G. COVID-19: a challenge for oncology services. Oncoimmunology 9, 1760686 (2020).
Weston, S. et al. Broad anti-coronaviral activity of FDA approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo. J. Virol. https://doi.org/10.1128/JVI.01218-20 (2020).
García-Martínez, E. et al. Trial Watch: immunostimulation with recombinant cytokines for cancer therapy. Oncoimmunology 7, e1433982 (2018).
Lu, H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends 14, 69–71 (2020).
Sheahan, T. P. et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon β against MERS-CoV. Nat. Commun. 11, 222 (2020).
Smaldone, G. C. Repurposing of gamma interferon via inhalation delivery. Adv. Drug Deliv. Rev. 133, 87–92 (2018).
Hirsch, L., Zitvogel, L., Eggermont, A. & Marabelle, A. PD-Loma: a cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br. J. Cancer 120, 3–5 (2019).
Zhao, J. et al. Intranasal treatment with poly(I•C) protects aged mice from lethal respiratory virus infections. J. Virol. 86, 11416–11424 (2012).
Marabelle, A., Bergeron, C., Billaud, G., Mekki, Y. & Girard, S. Hemophagocytic syndrome revealing primary HHV-6 infection. J. Pediatr. 157, 511 (2010).
Liu, P. et al. Nivolumab treatment of relapsed/refractory Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults. Blood 135, 826–833 (2020).
Luo, J. et al. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 10, 1121–1128 (2020).
Xu, X. et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl Acad. Sci. USA 117, 10970–10975 (2020).
Guo, C. Single-cell analysis of two severe COVID-19 patients reveals a monocyte-associated and tocilizumab-responding cytokine storm. Nat. Commun. https://doi.org/10.1038/s41467-020-17834-w (2020).
Liao, M. et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 26, 842–844 (2020).
Michot, J.-M. et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann. Oncol. https://doi.org/10.1016/j.annonc.2020.03.300 (2020).
Zhang, X. et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 4, 1307–1310 (2020).
Moots, R. J. et al. Effect of tocilizumab on neutrophils in adult patients with rheumatoid arthritis: pooled analysis of data from phase 3 and 4 clinical trials. Rheumatology 56, 541–549 (2017).
Parkinson, J. RA therapy studied for COVID-19 does not meet endpoint. ContagionLive https://www.contagionlive.com/news/ra-therapy-studied-for-covid19-does-not-meet-endpoint (2020)
Mikkonen, L., Pihlajamaa, P., Sahu, B., Zhang, F. P. & Jänne, O. A. Androgen receptor and androgen-dependent gene expression in lung. Mol. Cell. Endocrinol. 317, 14–24 (2010).
Montopoli, M. et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann. Oncol. 31, 1040–1045 (2020).
Bennani, N. & Bennani-Baiti, I. M. Androgen deprivation therapy may constitute a more effective prophylactic than therapeutic strategy in COVID-19 patients. Ann. Oncol. https://doi.org/10.1016/j.annonc.2020.08.2095 (2020).
Amaravadi, R. K., Kimmelman, A. C. & Debnath, J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 9, 1167–1181 (2019).
Gautret, P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 56, 105949 (2020).
Boulware, D. R. et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N. Engl. J. Med. 383, 517–525 (2020).
Cavalcanti, A. B. et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2019014 (2020).
Gordon, D. E. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468 (2020).
Spicka, I. et al. Randomized phase III study (ADMYRE) of plitidepsin in combination with dexamethasone vs. dexamethasone alone in patients with relapsed/refractory multiple myeloma. Ann. Hematol. 98, 2139–2150 (2019).
Coleman, C. M. et al. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus fusion. J. Virol. 90, 8924–8933 (2016).
Sauvat, A. et al. On-target versus off-target effects of drugs inhibiting the replication of SARS-CoV-2. Cell Death Dis. 11, 656 (2020).
Zitvogel, L., Rusakiewicz, S., Routy, B., Ayyoub, M. & Kroemer, G. Immunological off-target effects of imatinib. Nat. Rev. Clin. Oncol. 13, 431–446 (2016).
Nabavi, S. F. et al. Lessons learned from SARS-CoV and MERS-CoV: FDA-approved Abelson tyrosine-protein kinase 2 inhibitors may help us combat SARS-CoV-2. Arch. Med. Sci. 16, 519–5521 (2020).
Treon, S. P. et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood 135, 1912–1915 (2020).
Barreira da Silva, R. et al. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 16, 850–858 (2015).
Bacigalupo, A. et al. Treatment of steroid resistant acute graft versus host disease with an anti-CD26 monoclonal antibody—Begelomab. Bone Marrow Transplant. 55, 1580–1587 (2020).
Iacobellis, G. COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res. Clin. Pract. 162, 108125 https://pubmed.ncbi.nlm.nih.gov/32224164/ (2020).
Galimberti, S. et al. The CoV-2 outbreak: how hematologists could help to fight Covid-19. Pharmacol. Res. 157, 104866 (2020).
Klug, F. et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24, 589–602 (2013).
Meziani, L., Robert, C., Mordant, P. & Deutsch, E. Low doses of radiation therapy increase the immunosuppressive profile of lung macrophages via IL-10 production and IFNγ/IL-6 suppression: a therapeutic strategy to counteract lung inflammation? Preprint at bioRxiv https://doi.org/10.1101/2020.05.11.077651 (2020).
Calabrese, E. J. & Dhawan, G. How radiotherapy was historically used to treat pneumonia: could it be useful today? Yale J. Biol. Med. 86, 555–570 (2013).
Powell, E. V. Roentgen therapy of lobar pneumonia. J. Am. Med. Assoc. 110, 19–22 (1938).
Kirsch, D. G., Diehn, M., Cucinotta, F. A. & Weichselbaum, R. Lack of supporting data make the risks of a clinical trial of radiation therapy as a treatment for COVID-19 pneumonia unacceptable. Radiother. Oncol. 147, 217–220 (2020).
Wei, W. et al. Radiotherapy workflow and protection procedures during the Coronavirus Disease 2019 (COVID-19) outbreak: experience of the Hubei Cancer Hospital in Wuhan, China. Radiother. Oncol. 148, 203–210 (2020).
Aghili, M., Ghalehtaki, R., Mousavi Darzikolaee, N., Jafari, F. & Moshtaghian, M. Radiotherapy and COVID-19: practical recommendations from Iran. Radiother. Oncol. 149, 70–71 (2020).
Sriwijitalai, W. & Wiwanitkit, V. COVID-19, radiotherapy and cancer. Radiother. Oncol. 149, 48–48 (2020).
Jiménez-Sousa, M. Á., Martínez, I., Medrano, L. M., Fernández-Rodríguez, A. & Resino, S. Vitamin D in human immunodeficiency virus infection: influence on immunity and disease. Front. Immunol. 9, 458 (2018).
Huang, F. et al. Identification of amitriptyline HCl, flavin adenine dinucleotide, azacitidine and calcitriol as repurposing drugs for influenza A H5N1 virus-induced lung injury. PLoS Pathog. 16, e1008341 (2020).
Cannell, J. J. et al. Epidemic influenza and vitamin D. Epidemiol. Infect. 134, 1129–1140 (2006).
Hribar, C. A., Cobbold, P. H. & Church, F. C. Potential role of vitamin D in the elderly to resist COVID-19 and to slow progression of Parkinson’s disease. Brain Sci. 10, 284 (2020).
Zhou, Y.-F., Luo, B.-A. & Qin, L.-L. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine (Baltimore) 98, e17252 (2019).
Jolliffe, D. A. et al. Vitamin D receptor genotype influences risk of upper respiratory infection. Br. J. Nutr. 120, 891–900 (2018).
Xu, J. et al. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 16, 7432–7438 (2017).
Keum, N. & Giovannucci, E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br. J. Cancer 111, 976–980 (2014).
Manson, J. E. et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 380, 33–44 (2019).
Wang, H. & Zhang, L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 21, e181 (2020).
Mannelli, C. Whose life to save? Scarce resources allocation in the COVID-19 outbreak. J. Med. Ethics 46, 364–366 (2020).
Richards, M., Anderson, M., Carter, P., Ebert, B. L. & Mossialos, E. The impact of the COVID-19 pandemic on cancer care. Nat. Cancer 1, 1–3 (2020).
Andreani, J. et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 145, 104228 (2020).
Liu, J. et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 6, 16 (2020).
Anastasiou, I. A., Eleftheriadou, I., Tentolouris, A., Tsilingiris, D. & Tentolouris, N. In vitro data of current therapies for SARS-CoV-2. Curr. Med. Chem. 27, 4542–4548 (2020).
Hoffmann, M. et al. Nafamostat mesylate blocks activation of SARS-CoV-2: New treatment option for COVID-19. Antimicrob. Agents Chemother. 64, e00754–20 (2020).
Wang, M. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30, 269–271 (2020).
Hansen, J. et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 369, 1010–1014 (2020).
Richardson, P. et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395, e30–e31 (2020).
Vankadari, N. & Wilce, J. A. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 9, 601–604 (2020).
Yang, N. & Shen, H.-M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int. J. Biol. Sci. 16, 1724–1731 (2020).
Inoue, Y. et al. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 81, 8722–8729 (2007).
Cavalli, G. et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2, e325–e331 (2020).
Huet, T. et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2, e393–e400 (2020).
Mulder, W. J. M., Ochando, J., Joosten, L. A. B., Fayad, Z. A. & Netea, M. G. Therapeutic targeting of trained immunity. Nat. Rev. Drug Discov. 18, 553–566 (2019).
Hamiel, U., Kozer, E. & Youngster, I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. J. Am. Med. Assoc. 323, 2340 (2020).
Stuart-Harris, R., Buckman, R., Starke, I. & Wiltshaw, E. Chlorpromazine, placebo and droperidol in the treatment of nausea and vomiting associated with cisplatin therapy. Postgrad. Med. J. 59, 500–502 (1983).
Gadina, M. et al. Protective effect of chlorpromazine on endotoxin toxicity and TNF production in glucocorticoid-sensitive and glucocorticoid-resistant models of endotoxic shock. J. Exp. Med. 173, 1305–1310 (1991).
Lee, W. Y. et al. Repositioning antipsychotic chlorpromazine for treating colorectal cancer by inhibiting sirtuin 1. Oncotarget 6, 27580–27595 (2015).
Cardoso, A. T., Nanji, L., Costa, J. & Vaz-Carneiro, A. [Analysis of the Cochrane Review: VITAMIN D supplementation for prevention of cancer in adults. Cochrane Database Syst. Rev. 2014, 6:CD007469]. Acta Med. Port. 27, 411–413 (2014).
Marik, P. E., Kory, P. & Varon, J. Does vitamin D status impact mortality from SARS-CoV-2 infection? Med. Drug. Discov. 6, 100041 (2020).
Izumiya, Y. et al. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension 47, 887–893 (2006).
Eschenhagen, T. et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 13, 1–10 (2011).
Pinder, M. C., Duan, Z., Goodwin, J. S., Hortobagyi, G. N. & Giordano, S. H. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J. Clin. Oncol. 25, 3808–3815 (2007).
Von Hoff, D. D. et al. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 91, 710–717 (1979).
Sawyer, D. B., Zuppinger, C., Miller, T. A., Eppenberger, H. M. & Suter, T. M. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1β and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation 105, 1551–1554 (2002).
te Poele, R. H., Okorokov, A. L., Jardine, L., Cummings, J. & Joel, S. P. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 62, 1876–1883 (2002).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
Poole, B. B., Hamilton, L. A., Brockman, M. M. & Byrd, D. C. Interstitial pneumonitis from treatment with gemcitabine. Hosp. Pharm. 49, 847–850 (2014).
Olson, P. N., Schultheiss, P. & Seim, H. B. III. Clinical and laboratory findings associated with actual or suspected azoospermia in dogs: 18 cases (1979–1990). J. Am. Vet. Med. Assoc. 201, 478–482 (1992).
Shi, K., Wang, D., Cao, X. & Ge, Y. Endoplasmic reticulum stress signaling is involved in mitomycin c (MMC)-induced apoptosis in human fibroblasts via PERK pathway. PLoS ONE 8, e59330 (2013).
Perros, F. et al. Mitomycin-induced pulmonary veno-occlusive disease: evidence from human disease and animal models. Circulation 132, 834–847 (2015).
Smith, M. R. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology 63, 742–745 (2004).
Guise, T. A. Bone loss and fracture risk associated with cancer therapy. Oncologist 11, 1121–1131 (2006).
Israeli, R. S., Ryan, C. W. & Jung, L. L. Managing bone loss in men with locally advanced prostate cancer receiving androgen deprivation therapy. J. Urol. 179, 414–423 (2008).
Bjarnason, N. H., Hitz, M., Jorgensen, N. R. & Vestergaard, P. Adverse bone effects during pharmacological breast cancer therapy. Acta Oncol. 47, 747–754 (2008).
Chandra, A. et al. PTH1-34 alleviates radiotherapy-induced local bone loss by improving osteoblast and osteocyte survival. Bone 67, 33–40 (2014).
Chen, Z., Wu, Z. & Ning, W. Advances in molecular mechanisms and treatment of radiation-induced pulmonary fibrosis. Transl. Oncol. 12, 162–169 (2019).
Ewer, S. M. & Ewer, M. S. Cardiotoxicity profile of trastuzumab. Drug Saf. 31, 459–467 (2008).
Mohan, N., Jiang, J., Dokmanovic, M. & Wu, W. J. Trastuzumab-mediated cardiotoxicity: current understanding, challenges, and frontiers. Antib Ther 1, 13–17 (2018).
Xu, R. & Zhu, J. Comment on: Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: meta-analysis of 153 cohorts with 15,713 patients: meta-analysis of incidence and risk factors of EGFR-TKI pneumonitis in NSCLC. Lung Cancer 127, 167 (2019).
Kochupurakkal, N. M. et al. Blockade of the programmed death-1 (PD1) pathway undermines potent genetic protection from type 1 diabetes. PLoS ONE 9, e89561 (2014).
Kähler, K. C. & Hauschild, A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J. Dtsch. Dermatol. Ges. 9, 277–286 (2011).
Giavridis, T. et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738 (2018).
Soefje, S. A., Karnad, A. & Brenner, A. J. Common toxicities of mammalian target of rapamycin inhibitors. Target. Oncol. 6, 125–129 (2011).
Shi, L., Tang, J., Tong, L. & Liu, Z. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: a systematic review and meta-analysis of clinical trials. Lung Cancer 83, 231–239 (2014).
Busaidy, N. L. et al. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J. Clin. Oncol. 30, 2919–2928 (2012).
Morelon, E. et al. Characteristics of sirolimus-associated interstitial pneumonitis in renal transplant patients. Transplantation 72, 787–790 (2001).
Schmidinger, M. et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 26, 5204–5212 (2008).
Chu, T. F. et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370, 2011–2019 (2007).
Mukai, S. et al. Macrolides sensitize EGFR-TKI-induced non-apoptotic cell death via blocking autophagy flux in pancreatic cancer cell lines. Int. J. Oncol. 48, 45–54 (2016).
Abdel-Hamid, N. I., El-Azab, M. F. & Moustafa, Y. M. Macrolide antibiotics differentially influence human HepG2 cytotoxicity and modulate intrinsic/extrinsic apoptotic pathways in rat hepatocellular carcinoma model. Naunyn Schmiedebergs Arch. Pharmacol. 390, 379–395 (2017).
Qiao, X., Wang, X., Shang, Y., Li, Y. & Chen, S.-Z. Azithromycin enhances anticancer activity of TRAIL by inhibiting autophagy and up-regulating the protein levels of DR4/5 in colon cancer cells in vitro and in vivo. Cancer Commun. (Lond.) 38, 43 (2018).
Moriya, S. et al. Macrolide antibiotics block autophagy flux and sensitize to bortezomib via endoplasmic reticulum stress-mediated CHOP induction in myeloma cells. Int. J. Oncol. 42, 1541–1550 (2013).
Li, F. et al. Azithromycin effectively inhibits tumor angiogenesis by suppressing vascular endothelial growth factor receptor 2-mediated signaling pathways in lung cancer. Oncol. Lett. 14, 89–96 (2017).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8 (2020).
Kim, E. L. et al. Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro-oncol. 12, 389–400 (2010).
Jiang, P. D. et al. Cell growth inhibition, G2/M cell cycle arrest, and apoptosis induced by chloroquine in human breast cancer cell line Bcap-37. Cell. Physiol. Biochem. 22, 431–440 (2008).
Kim, M.-Y. et al. Combination therapy with a PI3K/mTOR dual inhibitor and chloroquine enhances synergistic apoptotic cell death in Epstein-Barr virus-infected gastric cancer cells. Mol. Cells 42, 448–459 (2019).
Yang, A. et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 4, 905–913 (2014).
Park, E. J. et al. Chloroquine enhances TRAIL-mediated apoptosis through up-regulation of DR5 by stabilization of mRNA and protein in cancer cells. Sci. Rep. 6, 22921 (2016).
Zhu, B. et al. Inhibition of autophagy with chloroquine enhanced sinoporphyrin sodium mediated photodynamic therapy-induced apoptosis in human colorectal cancer cells. Int. J. Biol. Sci. 15, 12–23 (2019).
Fukuda, T. et al. The anti-malarial chloroquine suppresses proliferation and overcomes cisplatin resistance of endometrial cancer cells via autophagy inhibition. Gynecol. Oncol. 137, 538–545 (2015).
Jarauta, V. et al. Inhibition of autophagy with chloroquine potentiates carfilzomib-induced apoptosis in myeloma cells in vitro and in vivo. Cancer Lett. 382, 1–10 (2016).
Hounjet, J. et al. The anti-malarial drug chloroquine sensitizes oncogenic NOTCH1 driven human T-ALL to γ-secretase inhibition. Oncogene 38, 5457–5468 (2019).
Jiang, P.-D. et al. [Effects of chloroquine diphosphate on proliferation and apoptosis of human leukemic K562 cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 16, 768–771 (2008).
Masud Alam, M. et al. Inhibition of autophagy by chloroquine induces apoptosis in primary effusion lymphoma in vitro and in vivo through induction of endoplasmic reticulum stress. Apoptosis 21, 1191–1201 (2016).
Lakhter, A. J. et al. Chloroquine promotes apoptosis in melanoma cells by inhibiting BH3 domain-mediated PUMA degradation. J. Invest. Dermatol. 133, 2247–2254 (2013).
Cournoyer, S. et al. GX15-070 (Obatoclax), a Bcl-2 family proteins inhibitor engenders apoptosis and pro-survival autophagy and increases chemosensitivity in neuroblastoma. BMC Cancer 19, 1018 (2019).
Wang, W. et al. Hydroxychloroquine enhances the antitumor effects of BC001 in gastric cancer. Int. J. Oncol. 55, 405–414 (2019).
Saleem, A. et al. Effect of dual inhibition of apoptosis and autophagy in prostate cancer. Prostate 72, 1374–1381 (2012).
Hall, E. A. et al. Novel organometallic chloroquine derivative inhibits tumor growth. J. Cell. Biochem. 119, 5921–5933 (2018).
Levy, J. M. M., Towers, C. G. & Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528–542 (2017).
Rosenfeld, M. R. et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy 10, 1359–1368 (2014).
Xu, X. et al. Autophagy is essential for effector CD8+ T cell survival and memory formation. Nat. Immunol. 15, 1152–1161 (2014).
Choy, K.-T. et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 178, 104786 (2020).
Kariya, R. et al. HIV protease inhibitor Lopinavir induces apoptosis of primary effusion lymphoma cells via suppression of NF-κB pathway. Cancer Lett. 342, 52–59 (2014).
Paskas, S. et al. Lopinavir-NO, a nitric oxide-releasing HIV protease inhibitor, suppresses the growth of melanoma cells in vitro and in vivo. Invest. New Drugs 37, 1014–1028 (2019).
Maksimovic-Ivanic, D. et al. The NO-modified HIV protease inhibitor as a valuable drug for hematological malignancies: Role of p70S6K. Leuk. Res. 39, 1088–1095 (2015).
Selvakumaran, M., Amaravadi, R. K., Vasilevskaya, I. A. & O’Dwyer, P. J. Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin. Cancer Res. 19, 2995–3007 (2013).
Meier-Stephenson, V., Riemer, J. & Narendran, A. The HIV protease inhibitor, nelfinavir, as a novel therapeutic approach for the treatment of refractory pediatric leukemia. Onco Targets Ther. 10, 2581–2593 (2017).
Wang, X. et al. Nitazoxanide, an antiprotozoal drug, inhibits late-stage autophagy and promotes ING1-induced cell cycle arrest in glioblastoma. Cell Death Dis. 9, 1032 (2018).
Ripani, P. et al. Thiazolides promote G1 cell cycle arrest in colorectal cancer cells by targeting the mitochondrial respiratory chain. Oncogene 39, 2345–2357 (2020).
Müller, J. et al. Thiazolides inhibit growth and induce glutathione-S-transferase Pi (GSTP1)-dependent cell death in human colon cancer cells. Int. J. Cancer 123, 1797–1806 (2008).
Fan-Minogue, H. et al. A c-Myc activation sensor-based high-throughput drug screening identifies an antineoplastic effect of nitazoxanide. Mol. Cancer Ther. 12, 1896–1905 (2013).
Senkowski, W. et al. Three-dimensional cell culture-based screening identifies the anthelmintic drug nitazoxanide as a candidate for treatment of colorectal cancer. Mol. Cancer Ther. 14, 1504–1516 (2015).
Chen, X. et al. The molecular aspect of antitumor effects of protease inhibitor nafamostat mesylate and its role in potential clinical applications. Front. Oncol. 9, 852 (2019).
Haruki, K. et al. Inhibition of nuclear factor-κB enhances the antitumor effect of tumor necrosis factor-α gene therapy for hepatocellular carcinoma in mice. Surgery 154, 468–478 (2013).
Iwase, R. et al. Combination chemotherapy of nafamostat mesylate with gemcitabine for gallbladder cancer targeting nuclear factor-κB activation. J. Surg. Res. 184, 605–612 (2013).
Gocho, T. et al. Combination chemotherapy of serine protease inhibitor nafamostat mesilate with oxaliplatin targeting NF-κB activation for pancreatic cancer. Cancer Lett. 333, 89–95 (2013).
Lu, Y.-X. et al. Inhibition of the NF-κB pathway by nafamostat mesilate suppresses colorectal cancer growth and metastasis. Cancer Lett. 380, 87–97 (2016).
Srinivas, P., Sacha, G. & Koval, C. Antivirals for COVID-19. Cleve. Clin. J. Med. https://doi.org/10.3949/ccjm.87a.ccc030 (2020).
Thulasiraman, P. et al. Neuraminidase 1 regulates proliferation, apoptosis and the expression of Cadherins in mammary carcinoma cells. Mol. Cell. Biochem. 462, 207–215 (2019).
Allison Logan, S., Brissenden, A. J., Szewczuk, M. R. & Neufeld, R. J. Combinatorial and sequential delivery of gemcitabine and oseltamivir phosphate from implantable poly(d,l-lactic-co-glycolic acid) cylinders disables human pancreatic cancer cell survival. Drug Des. Devel. Ther. 11, 2239–2250 (2017).
de Oliveira, J. T. et al. Anti-influenza neuraminidase inhibitor oseltamivir phosphate induces canine mammary cancer cell aggressiveness. PLoS ONE 10, e0121590 (2015).
Zhang, W. F., Stephen, P., Thériault, J.-F., Wang, R. & Lin, S.-X. Novel coronavirus polymerase and nucleotidyl-transferase structures: potential to target new outbreaks. J. Phys. Chem. Lett. 11, 4430–4435 (2020).
Moura, M. D. G., Haddad, J. P. A., Senna, M. I. B., Ferreira e Ferreira, E. & Mesquita, R. A. A new topical treatment protocol for oral hairy leukoplakia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 110, 611–617 (2010).
Shaw, M. M., Gürr, W. K., Watts, P. A., Littler, E. & Field, H. J. Ganciclovir and penciclovir, but not acyclovir, induce apoptosis in herpes simplex virus thymidine kinase-transformed baby hamster kidney cells. Antivir. Chem. Chemother. 12, 175–186 (2001).
Volpin, F. et al. Use of an anti-viral drug, Ribavirin, as an anti-glioblastoma therapeutic. Oncogene 36, 3037–3047 (2017).
Wang, G. et al. Targeting eIF4E inhibits growth, survival and angiogenesis in retinoblastoma and enhances efficacy of chemotherapy. Biomed. Pharmacother. 96, 750–756 (2017).
Kentsis, A., Topisirovic, I., Culjkovic, B., Shao, L. & Borden, K. L. B. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc. Natl Acad. Sci. USA 101, 18105–18110 (2004).
Dunn, L. A. et al. Phase I study of induction chemotherapy with afatinib, ribavirin, and weekly carboplatin and paclitaxel for stage IVA/IVB human papillomavirus-associated oropharyngeal squamous cell cancer. Head Neck 40, 233–241 (2018).
Xi, C. et al. Inhibition of eukaryotic translation initiation factor 4E is effective against chemo-resistance in colon and cervical cancer. Biochem. Biophys. Res. Commun. 503, 2286–2292 (2018).
Tan, J., Ye, J., Song, M., Zhou, M. & Hu, Y. Ribavirin augments doxorubicin’s efficacy in human hepatocellular carcinoma through inhibiting doxorubicin-induced eIF4E activation. J. Biochem. Mol. Toxicol. https://doi.org/10.1002/jbt.22007 (2018).
Teng, L. et al. Anti-tumor effect of ribavirin in combination with interferon-α on renal cell carcinoma cell lines in vitro. Cancer Cell Int. 14, 63 (2014).
Dominguez-Gomez, G. et al. Growth inhibition and transcriptional effects of ribavirin in lymphoma. Oncol. Rep. 42, 1248–1256 (2019).
Urtishak, K. A. et al. Targeting EIF4E signaling with ribavirin in infant acute lymphoblastic leukemia. Oncogene 38, 2241–2262 (2019).
Casaos, J. et al. Ribavirin as a potential therapeutic for atypical teratoid/rhabdoid tumors. Oncotarget 9, 8054–8067 (2018).
Shen, X. et al. Antiviral drug ribavirin targets thyroid cancer cells by inhibiting the eIF4E-β-catenin axis. Am. J. Med. Sci. 354, 182–189 (2017).
Cao, J., Sun, X., Zhang, X. & Chen, D. Inhibition of eIF4E cooperates with chemotherapy and immunotherapy in renal cell carcinoma. Clin. Transl. Oncol. 20, 761–767 (2018).
Kini, G. D., Robins, R. K. & Avery, T. L. Synthesis and antitumor activity of ribavirin imidates. A new facile synthesis of ribavirin amidine (1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamidine hydrochloride). J. Med. Chem. 32, 1447–1449 (1989).
Chen, J., Xu, X. & Chen, J. Clinically relevant concentration of anti-viral drug ribavirin selectively targets pediatric osteosarcoma and increases chemosensitivity. Biochem. Biophys. Res. Commun. 506, 604–610 (2018).
Laurent, N. et al. Effects of the proteasome inhibitor ritonavir on glioma growth in vitro and in vivo. Mol. Cancer Ther. 3, 129–136 (2004).
Moawad, E. Y. Identifying the optimal dose of ritonavir in the treatment of malignancies. Metab. Brain Dis. 29, 533–540 (2014).
Bandiera, E. et al. The HIV-protease inhibitor saquinavir reduces proliferation, invasion and clonogenicity in cervical cancer cell lines. Oncol. Lett. 12, 2493–2500 (2016).
Maggiorella, L. et al. Combined radiation sensitizing and anti-angiogenic effects of ionizing radiation and the protease inhibitor ritonavir in a head and neck carcinoma model. Anticancer Res. 25, 4357–4362 (2005).
Ikezoe, T. et al. HIV-1 protease inhibitor, ritonavir: a potent inhibitor of CYP3A4, enhanced the anticancer effects of docetaxel in androgen-independent prostate cancer cells in vitro and in vivo. Cancer Res. 64, 7426–7431 (2004).
Dalva-Aydemir, S. et al. Targeting the metabolic plasticity of multiple myeloma with FDA-approved ritonavir and metformin. Clin. Cancer Res. 21, 1161–1171 (2015).
Dewan, M. Z. et al. An HIV protease inhibitor, ritonavir, targets the nuclear factor-κB and inhibits the tumor growth and infiltration of EBV-positive lymphoblastoid B cells. Int. J. Cancer 124, 622–629 (2009).
Pati, S. et al. Antitumorigenic effects of HIV protease inhibitor ritonavir: inhibition of Kaposi sarcoma. Blood 99, 3771–3779 (2002).
Srirangam, A. et al. Effects of HIV protease inhibitor ritonavir on Akt-regulated cell proliferation in breast cancer. Clin. Cancer Res. 12, 1883–1896 (2006).
Isono, M., Sato, A., Asano, T., Okubo, K. & Asano, T. Delanzomib interacts with ritonavir synergistically to cause endoplasmic reticulum stress in renal cancer cells. Anticancer Res. 38, 3493–3500 (2018).
Gaedicke, S. et al. Antitumor effect of the human immunodeficiency virus protease inhibitor ritonavir: induction of tumor-cell apoptosis associated with perturbation of proteasomal proteolysis. Cancer Res. 62, 6901–6908 (2002).
Wang, X. et al. The anti-influenza virus drug arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 6, 28 (2020).
Acknowledgements
L. Z. and G. K. are supported by the Ligue contre le Cancer (équipe labellisée); Agence Nationale de la Recherche (ANR)–Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085), Institut National du Cancer (INCa); INSERM (HTE); Institut Universitaire de France; LeDucq Foundation; LabEx Immuno-Oncology (ANR-18-IDEX-0001); RHU Torino Lumière; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). A. M. has received pre-clinical and clinical cancer research funding from the Fondation MSD Avenir and Malakoff Médéric. L.D. has received support by the Philanthropia Fondation. L. D. and L. Z. are supported by the Gustave Roussy–sponsored clinical study on COVID-19 (ONCOVID; NCT04341207 supported by the Gustave Roussy Fondation, the Dassault family and Malakoff Humanis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Derosa, L., Melenotte, C., Griscelli, F. et al. The immuno-oncological challenge of COVID-19. Nat Cancer 1, 946–964 (2020). https://doi.org/10.1038/s43018-020-00122-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-020-00122-3
This article is cited by
-
Gut OncoMicrobiome Signatures (GOMS) as next-generation biomarkers for cancer immunotherapy
Nature Reviews Clinical Oncology (2023)
-
Cancer management during the COVID-19 world pandemic
Cancer Immunology, Immunotherapy (2023)
-
A Machine Learning Approach to Identify Potential miRNA-Gene Regulatory Network Contributing to the Pathogenesis of SARS-CoV-2 Infection
Biochemical Genetics (2023)
-
Learning from cancer to address COVID-19
Biologia Futura (2023)
-
Excess mortality associated with the COVID-19 pandemic in Latvia: a population-level analysis of all-cause and noncommunicable disease deaths in 2020
BMC Public Health (2022)