Abstract
Harnessing the immune system by blocking the programmed cell death protein 1 (PD-1) pathway has been a major breakthrough in non-small-cell lung cancer treatment. Nonetheless, many patients fail to respond to PD-1 inhibition. Using three syngeneic models, we demonstrate that short-term starvation synergizes with PD-1 blockade to inhibit lung cancer progression and metastasis. This antitumor activity was linked to a reduction in circulating insulin-like growth factor 1 (IGF-1) and a downregulation of IGF-1 receptor (IGF-1R) signaling in tumor cells. A combined inhibition of IGF-1R and PD-1 synergistically reduced tumor growth in mice. This effect required CD8 cells, boosted the intratumoral CD8/Treg ratio and led to the development of tumor-specific immunity. In patients with non-small-cell lung cancer, high plasma levels of IGF-1 or high IGF-1R expression in tumors was associated with resistance to anti-PD-1–programmed death-ligand 1 immunotherapy. In conclusion, our data strongly support the clinical evaluation of IGF-1 modulators in combination with PD-1 blockade.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Brahmer, J. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
Horn, L. et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 379, 2220–2229 (2018).
Melero, I. et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Cancer 15, 457–472 (2015).
Ward, P. S. & Thompson, C. B. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21, 297–308 (2012).
Nencioni, A., Caffa, I., Cortellino, S. & Longo, V. D. Fasting and cancer: molecular mechanisms and clinical application. Nat. Rev. Cancer 18, 707–719 (2018).
Lee, C. et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 70, 1564–1572 (2010).
Lee, C. et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 4, 124ra27 (2012).
Di Biase, S. et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell 30, 136–146 (2016).
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016).
Tang, H. et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 29, 285–296 (2016).
Gibbons, D. L. et al. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev. 23, 2140–2151 (2009).
Bleau, A. M. et al. New syngeneic inflammatory-related lung cancer metastatic model harboring double KRAS/WWOX alterations. Int. J. Cancer 135, 2516–2527 (2014).
Trojan, J. et al. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science 259, 94–97 (1993).
Gable, K. L. Diarylureas are small-molecule inhibitors of insulin-like growth factor I receptor signaling and breast cancer cell growth. Mol. Cancer Ther. 5, 1079–1086 (2006).
Willcox, B. J. et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann. NY Acad. Sci. 1114, 434–455 (2007).
Mattison, J. A. et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321 (2012).
de Braud, F. et al. Abstract B022: metabolic and immunologic effects of the fasting mimicking diet in cancer patients. in AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics. Mol. Cancer Ther. 17(1 Suppl), Abstract nr B022 (2018).
Dey, M. et al. Heme oxygenase-1 protects regulatory T cells from hypoxia-induced cellular stress in an experimental mouse brain tumor model. J. Neuroimmunol. 266, 33–42 (2014).
Idelman, G. et al. The role of the IGF system in T-lymphocyte activation. Cancer Res. 67, 4392 (2007).
Bilbao, D., Luciani, L., Johannesson, B., Piszczek, A. & Rosenthal, N. Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease. EMBO Mol. Med. 6, 1423–1435 (2014).
Miyagawa, I. et al. Induction of regulatory T cells and its regulation with insulin-like growth factor/insulin-like growth factor binding protein-4 by human mesenchymal stem cells. J. Immunol. 199, 1616–1625 (2017).
Ni, F. et al. IGF-1 promotes the development and cytotoxic activity of human NK cells. Nat. Commun. 4, 1479 (2013).
Spadaro, O. et al. IGF1 shapes macrophage activation in response to immunometabolic challenge. Cell Rep. 19, 225–234 (2017).
Bach, L. A. Endothelial cells and the IGF system. J. Mol. Endocrinol. 54, R1–R13 (2015).
Nakagawa, M. et al. Clinical significance of IGF1R expression in nonsmall-cell lung cancer. Clin. Lung Cancer 13, 136–142 (2012).
Yu, H. et al. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J. Natl Cancer Inst. 91, 151–156 (1999).
Langer, C. J. et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 32, 2059–2066 (2014).
Ramalingam, S. S. et al. Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 29, 4574–4580 (2011).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Luis-Ravelo, D. et al. A gene signature of bone metastatic colonization sensitizes for tumor-induced osteolysis and predicts survival in lung cancer. Oncogene 33, 5090–5099 (2014).
Antoń, I. et al. Receptor of activated protein C promotes metastasis and correlates with clinical outcome in lung adenocarcinoma. Am. J. Respir. Crit. Care Med. 186, 96–105 (2012).
Ajona, D. et al. Blockade of the complement C5a/C5aR1 axis impairs lung cancer bone metastasis by CXCL16-mediated effects. Am. J. Respir. Crit. Care Med. 197, 1164–1176 (2018).
Ezponda, T. et al. The oncoprotein SF2/ASF promotes non-small cell lung cancer survival by enhancing survivin expression. Clin. Cancer Res. 16, 4113–4125 (2010).
Ajona, D. et al. A combined PD-1/C5a blockade synergistically protects against lung cancer growth and metastasis. Cancer Discov. 7, 694–703 (2017).
Acknowledgements
We thank C. Zandueta and O. Rogero for technical assistance, and J. M. Kurie (The University of Texas MD Anderson Cancer Center, Houston, TX) for gifting the 393P cells. This study was supported by the Foundation for Applied Medical Research (FIMA), CIBERONC (CB16/12/00443 and CB16/12/00364), Fundación Científica de la Asociación Española Contra el Cáncer, Fundación Ramón Areces, Juan Serrano, Instituto de Salud Carlos III–Fondo de Investigación Sanitaria–Fondo Europeo de Desarrollo Regional ‘Una manera de hacer Europa’ (FEDER; PI17/00411, PI16/01352, PI16/01821 and AC14/00034), La Caixa Foundation, Caja Navarra Foundation and the Spanish Ministry of Economy and Competitiveness (SAF2015-71606R, SAF2016-78568-R and RTI 2018-094507-B-100). F.E. is funded by a predoctoral fellowship from the Asociación de Amigos de la Universidad de Navarra and from La Caixa Foundation. S.O.-E. was funded by a predoctoral fellowship from the Asociación de Amigos de la Universidad de Navarra and is now supported by an FPU fellowship. M.F.S. is supported by a Miguel Servet type I contract from Instituto de Salud Carlos III–Fondo de Investigación Sanitaria.
Author information
Authors and Affiliations
Contributions
D.Ajona and R.P. supervised the study and were responsible for the study conception and design. J.M.L.-P., M.F.S., A.G., J.L.P.-G., I.G.-B., I.E.-S. and C.E.A. provided clinical samples. D.Ajona, S.O.-E., F.E., K.V., Y.S., S.V., C.S. and F.L. performed the in vivo studies. K.V. and C.B. prepared the viral vectors and the stable clones. D.Ajona, A.C. and M.R. were responsible of the Luminex analysis. D.Ajona and A.R. carried out the immunohistochemistry experiments. T.L. and J.J.L. performed the ELISpot assay. D.Alignani optimized and supervised the flow cytometry experiments. D.Ajona, I.M. C.B., A.R. and S.V. carried out the mRNA and protein expression analyses. D.Ajona, R.P., T.L., J.J.L., F.L., A.C., M.R., I.E.-S., M.F.S., C.E.A. and L.M.M. were responsible for the analysis and interpretation of the data. D.Ajona, M.F.S., I.E.-S. and R.P. performed the statistical analysis. D.Ajona and R.P. wrote the manuscript. All authors critically reviewed the manuscript and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
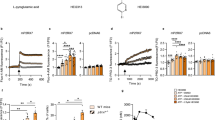
Extended Data Fig. 1 STS/anti-PD-1 treatment impairs 393P and LLC tumor growth.
a, Individual follow-up of 393P tumor volumes in mice treated with anti-PD-1 (n=10 mice), STS (n=10 mice), the combination of both (n=8 mice) or vehicle (Control; n=11 mice). b, Individual follow-up of LLC tumor volumes in mice treated with anti-PD-1 (n=8 mice), STS (n=6 mice), the combination of both (n=6 mice) or vehicle (Control; n=8 mice). Numerical source data are provided as a source data file.
Extended Data Fig. 2 Effects of STS/anti-PD-1 combination in LLC tumor immune infiltrate.
a, Flow cytometric analysis of the proportion of intratumoral CD4, NK (NK1.1) and B (CD19) cells at day 18 after subcutaneous inoculation of LLC cells (Control, n=8 tumors; anti-PD-1, n=8 tumors; STS, n=5 tumors; STS/anti-PD-1, n=7 tumors). b, Expression of PD-1 in tumor-infiltrating CD4 T cells of the experiment shown in section a. Data are expressed as the mean fluorescence intensity (MFI) ± s.e.m. c, Flow cytometric analysis of the proportion of intratumoral total MDSCs, monocytic MDSCs (M-MDSCs; CD11b/Ly6C+/Ly6Glow), granulocytic MDSCs (G-MDSC; CD11b/Ly6C+/Ly6G+), macrophages (F4/80) and DCs (CD11c). The number of tumors per group was: Control, n=7 tumors; anti-PD-1, n=8 tumors; STS, n=5 tumors; STS/anti-PD-1 combination, n=7 tumors. Data are expressed as the mean of the percentage of total leukocytes (CD45) ± s.e.m. In all cases, the statistical significance of the differences were evaluated using the two-sided Kruskal-Wallis test with the Mann-Whitney U-test as the post hoc test. Numerical source data are provided as a source data file.
Extended Data Fig. 3 Effects of STS/anti-PD-1 combination in the proportion of immune circulating cells from LLC tumor-bearing mice.
Flow cytometric analysis of the proportion of peripheral blood CD8, CD4, Treg cells, NK (NK1.1) and B (CD19) cells in mice after inoculation of LLC cells and two cycles of 48 hours of STS (day 12 after tumor implantation) (Control, n=6 mice; anti-PD-1, n=6 mice; STS, n=5 mice; STS/anti-PD-1, n=6 mice). Data are expressed as the percentage of total lymphocytes except for Treg cells, which are expressed as the percentage of total CD4 T cells (means ± s.e.m.). The differences between experimental groups were analyzed using the two-sided Kruskal-Wallis test with the Mann-Whitney U-test as the post hoc test. Numerical source data are provided as a source data file.
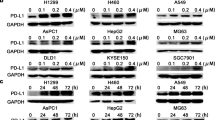
Extended Data Fig. 4 IGF-1 inhibits the increase in the LC3-II/LC3-I ratio induced by in vitro STS.
a, Cropping images from the western blot analysis of LC3-I and LC3-II in LLC cells cultured under normal or STS-like conditions for 48 hours. This experiment was performed twice with similar results. b, Cropping images from the western blot analysis of LC3-I and LC3-II in LLC cells cultured under normal or STS-like conditions in the presence of 1 µg ml–1 of rIGF-1 or vehicle for 48 and 72 hours. This experiment was performed once. Unprocessed images of blots are provided as source data file.
Extended Data Fig. 5 PQ401/anti-PD-1 treatment impairs 393P tumor growth.
Individual follow-up of 393P tumor volumes in mice treated with PQ401 (n=10 mice), anti-PD-1 (n=11 mice), or the combination of both (n=10 mice) or vehicle (Control; n=11 mice). Numerical source data are provided as a source data file.
Extended Data Fig. 6 Effects of PQ401/anti-PD-1 combination in LLC tumor immune infiltrate.
a, Flow cytometric analysis of the proportion of intratumoral total MDSCs, monocytic MDSCs (M-MDSCs; CD11b/Ly6C+/Ly6Glow), granulocytic MDSCs (G-MDSC; CD11b/Ly6C+/Ly6G+), macrophages (F4/80), DCs (CD11c), CD4 T, NK (NK1.1) and B (CD19) cells at day 19 after inoculation of LLC cells (n=8 tumors per group except for anti-PD-1 group in CD4 T, NK and B cells analyses; n=7). Data are expressed as the percentage of total leukocytes (CD45). b, Expression of the markers PD-1, GITR, and LAG-3 in tumor-infiltrating CD4 T cells. The number of tumors per group was: Control, n=8 tumors; anti-PD-1, n=7 tumors; PQ401, n=8 tumors; PQ401/anti-PD-1 combination, n=8 tumors. Data are expressed as the mean of fluorescence intensity (MFI) ± s.e.m. The differences between experimental groups were analyzed using the two-sided Kruskal-Wallis test with the Mann-Whitney U-test as the post hoc test. Numerical source data are provided as a source data file.
Extended Data Fig. 7 Effects of PQ401/anti-PD-1 combination in splenic immune populations from LLC tumor-bearing mice.
Flow cytometric analysis of the proportion of splenic CD8, CD4, B cells (CD19), NK (NK1.1) and Treg cells, MDSCs (total MDSCs, M-MDSCs, and G-MDSCs), macrophages, and DCs in tumor-bearing mice at day 19 after inoculation of LLC cells (n=8 mice per group). Data are expressed as the percentage of total leukocytes (CD45), except for Treg cells, which are expressed as the percentage of total CD4 T cells (means ± s.e.m.). The differences between experimental groups were analyzed using the two-sided Kruskal-Wallis test with the Mann-Whitney U-test as the post hoc test. No statistically significant differences were found. Numerical source data are provided as a source data file.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and Supplementary Figs. 1 and 2.
Source data
Source Data Fig. 1
Numerical source data
Source Data Fig. 2
Numerical source data
Source Data Fig. 3
Numerical source data
Source Data Fig. 4
Numerical source data
Source Data Fig. 5
Unprocessed Western blots
Source Data Fig. 5
Numerical source data
Source Data Fig. 6
Numerical source data
Source Data Fig. 7
Numerical source data
Source Data Extended Data Fig. 1
Numerical source data
Source Data Extended Data Fig. 2
Numerical source data
Source Data Extended Data Fig. 3
Numerical source data
Source Data Extended Data Fig. 4
Unprocessed Western blots
Source Data Extended Data Fig. 5
Numerical source data
Source Data Extended Data Fig. 6
Numerical source data
Source Data Extended Data Fig. 7
Numerical source data
Rights and permissions
About this article
Cite this article
Ajona, D., Ortiz-Espinosa, S., Lozano, T. et al. Short-term starvation reduces IGF-1 levels to sensitize lung tumors to PD-1 immune checkpoint blockade. Nat Cancer 1, 75–85 (2020). https://doi.org/10.1038/s43018-019-0007-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-019-0007-9
This article is cited by
-
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
Signal Transduction and Targeted Therapy (2024)
-
Fasting and fasting-mimicking conditions in the cancer immunotherapy era
Journal of Physiology and Biochemistry (2024)
-
Intermittent dietary methionine deprivation facilitates tumoral ferroptosis and synergizes with checkpoint blockade
Nature Communications (2023)
-
Fasting mimicking diet in mice delays cancer growth and reduces immunotherapy-associated cardiovascular and systemic side effects
Nature Communications (2023)
-
Dietary regulation in health and disease
Signal Transduction and Targeted Therapy (2022)