Abstract
Mycotoxins harm human and livestock health, while damaging economies. Here we reveal the changing threat of Fusarium head blight (FHB) mycotoxins in European wheat, using data from the European Food Safety Agency and agribusiness (BIOMIN, World Mycotoxin Survey) for ten years (2010–2019). We show persistent, high, single- and multi-mycotoxin contamination alongside changing temporal-geographical distributions, indicative of altering FHB disease pressure and pathogen populations, highlighting the potential synergistic negative health consequences and economic cost.
Similar content being viewed by others
Main
Mycotoxins are fungal secondary metabolites that are toxic to humans and livestock. They occur when fungal pathogens infect crops and contaminate their products. Mycotoxins contaminate 60–80% of crops, with 20% of global crops exceeding European Union (EU) legal food safety limits1.
Fusarium head blight (FHB) is a mycotoxigenic preharvest fungal disease of cereals, including wheat, which is a pivotal crop for human nutritional security2, livestock feed and international trade. Different Fusarium species within the FHB complex produce various mycotoxins that threaten human and livestock health, causing a wide range of toxic effects3. Therefore, the EU commission sets legal limits on FHB mycotoxin levels in food, but only guidelines for feed4. Grain exceeding the legal mycotoxin limit is downgraded from food to animal feed at an economic cost. Multi-mycotoxin synergisms are also an emerging concern for human and animal health5.
Effective FHB management requires an understanding of the current and changing scale of the disease. FHB symptoms on United Kingdom wheat have increased substantially since 2000 (Extended Data Fig. 1), but equivalent data on the incidence of FHB symptoms are lacking for Europe. However, FHB mycotoxins in the food and feed supply chains are monitored by the European Food Safety Agency (EFSA) and agribusiness (BIOMIN, World Mycotoxin Survey). These datasets have been used to assess global mycotoxin occurrence1,6, whereas FHB mycotoxin reports from individual European countries have been reviewed7. However, we require a clearer picture of the scale of the FHB mycotoxin problem in European food and feed wheat. Therefore, this work aimed to use these comprehensive mycotoxin datasets to quantify the changing threat from, and estimate the economic cost of, FHB in Europe. This spatiotemporal analysis of FHB mycotoxins in food and feed will provide quantitative insights into disease pressure, pathogen population composition and economic effect, thus facilitating future mycotoxin research and modelling, risk mitigation strategies and improved legislation.
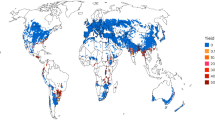
EFSA and BIOMIN data for the FHB mycotoxins deoxynivalenol (DON), fumonisin (FUM), zearalenone (ZEN) and T-2 were analysed for a ten-year period (2010–2019). DON occurrence (the percentage of wheat samples testing positive for DON) and levels (concentration of DON in a sample) were evaluated. In line with previous reports1,6 and the dominance of DON-producing Fusarium graminearum and F. culmorum on European wheat8, DON was the most common FHB mycotoxin in European food and feed wheat (Fig. 1a). European countries showed a positive correlation between food and feed, both in DON occurrence and levels (Extended Data Fig. 2a,b). This finding was as expected because wheat is divided into the food or feed streams postharvest based on quality (for example, protein content). If a geographic region experiences high disease pressure, this will increase DON in wheat throughout that region, regardless of whether the grain will later be designated as food or feed. DON occurrence and levels were lower in food wheat than in feed wheat. This suggests that supply chain management, such as postharvest downgrading of grain exceeding the DON limit for human consumption to animal feed, works effectively to reduce DON entering the food stream. DON in feed wheat may therefore be a more realistic indicator of FHB pressure in the field.
a, Percentage samples containing the FHB mycotoxins T-2, DON, FUM and ZEN. Box plots: centre lines indicate the median; box limits indicate the 25th and 75th percentiles; whiskers indicate the range; outliers are represented by dots. N food = 3,467, 6,519, 588, 4,953 samples. N feed = 2,673, 6,547, 2,090, 5,388 samples. b, Mean DON concentration (µg kg−1). c, Rate of change in DON level (µg kg−1 yr−1). White indicates no change. Grey indicates insufficient data.
DON was detected in food wheat from all European countries studied (average occurrence 47%, Fig. 1a), being most common at higher latitudes, with 93–69% wheat contamination in Sweden, the United Kingdom and Denmark. The occurrence of DON in United Kingdom wheat was consistent with recorded incidence of FHB disease symptoms in the field over the same period9 (Extended Data Fig. 1). DON occurrence remained relatively stable in most countries over the decade. Concerningly, DON levels were moderately high in contaminated food wheat (mean 358 µg kg−1), 5% of samples exceeded the 750 µg kg−1 legal limit for cereals for direct human consumption and the highest DON concentration recorded was 14,505 µg kg−1. The highest ten-year mean DON concentrations were in Hungary (722 µg kg−1) and the Netherlands (670 µg kg−1) (Fig. 1b). These were attributable to ‘peak’ years of elevated DON levels in 2010, 2011, 2015 and 2019 in Hungary and 2012 in the Netherlands. Contrary to expectation, a modest negative correlation between DON occurrence and mean level was seen in individual European countries (Extended Data Fig. 2c). Countries where DON was more common tended to record lower DON concentrations. For example, Sweden, Denmark and the United Kingdom showed a high occurrence of DON but at low concentrations, perhaps reflecting effective food supply chain mitigation to keep levels within legal limits. Nonetheless, the omnipresence of DON in food and at higher concentrations during peak years necessitates continuous and responsive FHB management. This raises concerns of chronic dietary DON exposure affecting human health. Indeed, the EFSA estimates that European chronic dietary exposure to DON exceeds the tolerable daily intake for children and is high in adolescents and adults10.
The situation in feed wheat was more concerning. DON was again the most common FHB mycotoxin (Fig. 1a), detected annually in every country, but at a higher prevalence (64%) than in food. In contrast to food, there was a positive correlation between DON prevalence and concentration in feed (Extended Data Fig. 2d). The ten-year average concentration of DON in contaminated feed wheat was 858 µg kg−1, exceeding the limit for human consumption. Of this, 1.5% exceeded EU maximum guidance values for animal feed (8000 µg kg−1), with DON concentrations of up to 49,000 µg kg−1 detected. Lower-latitude countries showed the highest prevalence (93–85% in France, Italy and Slovenia) and mean DON levels (1,393–1,279 µg kg−1 in France, Hungary and Romania) (Fig. 1b). The lowest, but still relatively high, DON levels (312–216 µg kg−1) were Denmark, the United Kingdom and Czechia. European livestock are therefore continuously exposed to high DON levels in feed wheat, potentially with negative animal health consequences.
A geographical pattern of changing threat from DON in feed wheat has emerged over the last decade. DON levels increased in countries below ~47° N latitude (Portugal, France and Romania; at a rate of +362 to 148 µg kg−1 yr), but decreased in countries above this latitude (the Netherlands, Finland and Austria; −258 to 118 µg kg−1 yr−1) (Fig. 1c). This trend was attributable to higher DON levels in peak years in the early 2010s and lower-level peaks in the late 2010s in higher-latitude countries, compared to lower-latitude countries, which had lower levels in the early 2010s and high-level peaks in 2018 and 2019. Peaks years of DON concentrations correspond to reported European FHB epidemics (Romania 2019 (ref. 11), Italy 2016 (ref. 12), United Kingdom 2012 (ref. 9); Extended Data Fig. 1). Increasing DON levels in peak years in lower latitudes was suggestive of increasingly severe FHB epidemics. This could be attributable to changing agronomic practices and climate. The adoption of minimum tillage farming practices and maize cultivation is known to increase FHB disease by providing a substrate for Fusarium to overwinter and produce inoculum in the spring13,14. Wheat infection occurs during anthesis and is promoted by high humidity and temperatures15. Climate change is projected to cause earlier anthesis, coinciding with wetter, warmer weather, thus increasing the severity of FHB epidemics16. Indeed, countries with similar trends in DON levels also tended to have similar climates17 (Extended Data Fig. 3). There were exceptions to this pattern; whereas most temperate oceanic (Cfb) countries showed DON levels were decreasing, DON levels in France (also classified as temperate oceanic (Cfb)) were increasing, akin to countries with Mediterranean (Csa) climates. This highlights the need for access to finer geographic resolution mycotoxin records to account for countries with distinct internal climatic zones, but also the need to understand differences in non-climatic factors, such as agronomic practice or pathogen population structure, to help explain this variation. Interestingly, lower-latitude regions of Europe have also been predicted to be increasingly at risk from other cereal mycotoxins, due to host-crop range expansion and climate change18. Fusarium populations vary in fungicide sensitivity19, and therefore emergence of fungicide resistance could also be a contributing factor. Collectively, this highlights the need to monitor FHB mycotoxin outbreaks and fungicide resistance for improved forecasting and threat mitigation.
Although DON dominates, concern is growing about synergisms between co-occurring mycotoxins. We evaluated the risk of co-contamination with other FHB mycotoxins. It should be noted that wheat tested for DON was not necessarily also tested for all other mycotoxins, and thus non-detection of a co-contaminant could be due to absence or a lack of testing. Approximately 25% of food and 45% of feed wheat containing DON tested positive for other FHB mycotoxins. The percentage of samples co-contaminated was stable over the decade, but the mycotoxin profile changed and multiple co-contaminants became more frequent (Fig. 2a), with ZEN and T-2 being the most common co-contaminants in food and feed. The high co-occurrence of ZEN with DON was expected, as they are both produced by F. graminearum and F. culmorum20. FUM co-contamination suggested the additional presence of F. proliferatum or F. verticillioides21. F. verticillioides is common at lower latitudes in Europe and emerging at higher latitudes due to the warming climate and increased cultivation of maize at higher latitudes18,22,23,24. Since 2010, the relative proportion of other mycotoxins, including T-2, has increased. This suggests that the FHB pathogen population in Europe has changed, with the T-2-producing F. langsethiae or F. sporotrichioides increasingly coinfecting wheat crops alongside DON-producing species. F. langsethiae was reported in 2004 on oats, barley and wheat from higher latitudes in Europe (Austria, Czechia, Denmark, the United Kingdom, the Netherlands and Germany)25, but has since been found in wheat in Poland26, Sweden27 and as far south as Italy28. Co-contamination incidence varies between European countries (Fig. 2b). Surprisingly, the prevalence of co-contamination in a country was inconsistent between food and feed, perhaps reflecting inadequate testing for non-DON mycotoxins. Although sample size of non-DON mycotoxins increases year on year in the feed dataset, it does not in food data. The limited testing for other FHB mycotoxins compared to DON means that co-contaminants may go undetected, and so the levels of co-contamination we report may be an underestimate. Therefore, it is imperative that multi-mycotoxin screening is undertaken if we are to fully understand the changing threat from pathogens and mycotoxins.
Across Europe in 2010–2019 we estimated that 75 million tonnes of wheat29 (5% of food wheat) exceeded the 750 µg kg−1 DON limit. Downgrading to feed equates to a loss of approximately €3 billion (Extended Data Table 1). The percentage of food wheat exceeding the DON limit was highest in 2012 (10.7%), a known FHB epidemic year9,30,31, but the cost of DON downgrading was highest in 2015, when the difference in value between food and feed wheat was greatest (86.74€ per tonne)32. Our estimates do not include losses from reduced yields, other FHB mycotoxins, or the cost of fungicide applications and mycotoxin testing, meaning this economic cost is a fraction of the total impact of FHB.
We have shown that FHB mycotoxins are ubiquitous across Europe, with DON persistently detected in wheat, raising concerns about the health effects of chronic dietary exposure. DON concentrations were worryingly high in feed wheat and mycotoxin outbreaks were becoming more severe in lower-latitude regions of Europe, possibly attributable to agronomic and climatic changes. Although lower contamination in food suggests the EU legal limits have a positive effect, rigorous monitoring and outbreak-responsive management of FHB mycotoxins must continue to protect human and livestock health. Changing mycotoxin profiles, such as increasing co-contamination with DON and T-2, indicate shifting dynamics in FHB pathogen populations and could have synergistic negative health implications. Our conservative economic estimates demonstrate the significant cost of DON contamination in European wheat. Our study quantifies the threat and cost of FHB mycotoxins, which should inform projections of food security scenarios in future climates, supporting the legislation and implementation of appropriate mycotoxin mitigation strategies.
Methods
To quantify the threat of FHB toxins in European food and feed wheat, we analysed data on mycotoxins in grain commodities to identify mycotoxin (1) occurrence, (2) levels and (3) how these varied across Europe, over time (2009–2019). Data on food were sourced from the EFSA, in accordance with Regulation (EC) 1049/2001 regarding public access to documents. The methods used for sampling and analysis are described in Commission Regulation (EC) 401/2006. Data on feed were supplied by BIOMIN Holding, Austria. Data were analysed using Microsoft Excel for Microsoft 365 MSO (v.2202). Data were filtered to retain samples with (1) an origin country in geographical Europe (as defined by the United Nations33), (2) being identifiable as wheat and (3) sampled between 2010 and 2019. EFSA data were filtered to retain samples identifiable as suitable for food; BIOMIN data were already solely for feed. After filtering, the EFSA dataset consisted of 6,519 samples, of which 3,035 contained DON, and the BIOMIN feed dataset consisted of 6,547 samples, of which 4,213 contained DON. Data from all countries were included in the European summary statistics. As a criterion for selecting data from individual countries to be presented, we set a minimum requirement of three samples from each of three different years (minimum of nine samples in total from a country). DON throughout refers to DON excluding its derivatives, because data on these were not available in the BIOMIN dataset. Mean DON level was calculated for the samples that tested positive for DON. To analyse co-occurrence over the decade, the percentage of samples from all countries containing DON that also contained any combination of FUM, ZEN and T-2 was calculated. The prevalence of co-occurrence in each European country was calculated as the percentage of samples containing DON that also contained FUM, ZEN or T-2. Nivalenol was excluded from the co-occurrence analyses because data on nivalenol were present in the EFSA dataset, but not the BIOMIN dataset. Maps were generated in QGIS v.2.22.11 using base-map data from GADM v.4.1.
For economic cost analysis, the percentage of samples exceeding the 750 µg kg−1 DON legal limit for direct human consumption4 was calculated from the EFSA data, for each year. The total food wheat production (total soft plus durum wheat) of the EU-28 was sourced from Eurostat29. The tonnes exceeding the DON limit each year was estimated by multiplying the percentage of samples exceeding limit by the total food wheat production. The difference in value per tonne between food and feed wheat was calculated by subtracting the average feed wheat price in the EU-28 (€ per tonne) from the average bread and durum wheat price in the EU-28 (€ per tonne), with prices sourced from the European Commission32. Value lost by downgrading the tonnes exceeding the legal DON limit was calculated by multiplying the tonnes lost by the value per tonne. This was done for each year and summed to give the total value lost over the ten years (Extended Data Table 1).
where C denotes the cost of downgrading (€), P the percentage of samples above the 750 µg kg−1 legal limit for DON in food for direct human consumption (%) (EFSA) and W the total food wheat production of the EU-28 (tonnes)29. Vfood denotes the value of food wheat per tonne and Vfeed the value of feed wheat per tonne (€ per tonne)32.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Extended data are available for this paper. Correspondence and requests for materials should be addressed to N. Brown. All data are included in this article and its Supplementary Information files. Food raw data are publicly available on request from the EFSA in accordance with Regulation (EC) 1049/2001 regarding public access to documents. Feed raw data provided by BIOMIN are not publicly available due to them containing information that may be of commercial interest and therefore are subject to a confidentiality and non-disclosure agreement. DEFRA winter wheat survey data were provided by J. Turner, Fera Science Limited, and are available on request as data supporting their paper https://doi.org/10.1111/ppa.13433. Summary data derived from these raw data are available in the Source data.
References
Eskola, M. et al. Worldwide contamination of food-crops with mycotoxins: validity of the widely cited ‘FAO estimate’of 25%. Crit. Rev. Food Sci. Nutr. 60, 2773–2789 (2020).
Poole, N., Donovan, J. & Erenstein, O. Agri-nutrition research: revisiting the contribution of maize and wheat to human nutrition and health. Food Policy 100, 101976 (2020).
Polak-Śliwińska, M. & Paszczyk, B. Trichothecenes in food and feed, relevance to human and animal health and methods of detection: a systematic review. Molecules 26, 454 (2021).
European Commission. Commission Regulation (EC) 1881/2006. Official Journal of the European Union (19 December 2006).
Alassane-Kpembi, I. et al. Mycotoxins co-contamination: methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 57, 3489–3507 (2017).
Gruber-Dorninger, C., Jenkins, T. & Schatzmayr, G. Global mycotoxin occurrence in feed: a ten-year survey. Toxins 11, 375 (2019).
Luo, S., Du, H., Kebede, H., Liu, Y. & Xing, F. Contamination status of major mycotoxins in agricultural product and food stuff in Europe. Food Control 127, 108120 (2021).
Waalwijk, C. et al. Quantitative detection of Fusarium species in wheat using TaqMan. Eur. J. Plant Pathol. 110, 481–494 (2004).
Turner, J. A., Chantry, T., Taylor, M. C. & Kennedy, M. C. Changes in agronomic practices and incidence and severity of diseases in winter wheat in England and Wales between 1999 and 2019. Plant Pathol. 70, 1759–1778 (2021).
EFSA Panel on Contaminants in the Food Chain.Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 15, e04718 (2017).
Cotuna, O., Paraschivu, M., Sărățeanu, V., Partal, E. & Durău, C. C. Influence of Fusarium graminearum infection on the accumulation of mycotoxins in wheat grains. Preprints 2021, 2021060429 (2021).
Scarpino, V. & Blandino, M. Effects of durum wheat cultivars with different degrees of FHB susceptibility grown under different meteorological conditions on the contamination of regulated, modified and emerging mycotoxins. Microorg. 9, 408 (2021).
Edwards, S. G. & Jennings, P. Impact of agronomic factors on Fusarium mycotoxins in harvested wheat. Food Add. Contam. Part A 35, 2443–2454 (2018).
Wegulo, S. N. Factors influencing deoxynivalenol accumulation in small grain cereals. Toxins 4, 1157–1180 (2012).
Xu, X. M., Monger, W., Ritieni, A. & Nicholson, P. Effect of temperature and duration of wetness during initial infection periods on disease development, fungal biomass and mycotoxin concentrations on wheat inoculated with single, or combinations of, Fusarium species. Plant Pathol. 56, 943–956 (2007).
Madgwick, J. W. et al. Impacts of climate change on wheat anthesis and Fusarium ear blight in the UK. Eur. J. Plant Pathol. 130, 117–131 (2011).
MacLeod, A. & Korycinska, A. Detailing Köppen–Geiger climate zones at sub‐national to continental scale: a resource for pest risk analysis. EPPO Bulletin 49, 73–82 (2019).
Battilani, P. et al. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 6, 1–7 (2016).
Anderson, N. R. et al. Sensitivity of Fusarium graminearum to metconazole and tebuconazole fungicides before and after widespread use in wheat in the United States. Plant Health Prog. 21, 85–90 (2020).
Kokkonen, M., Ojala, L., Parikka, P. & Jestoi, M. Mycotoxin production of selected Fusarium species at different culture conditions. Int. J. Food Microbiol. 143, 17–25 (2010).
Cendoya, E., Chiotta, M. L., Zachetti, V., Chulze, S. N. & Ramirez, M. L. Fumonisins and fumonisin-producing Fusarium occurrence in wheat and wheat by products: a review. J. Cereal Sci. 80, 158–166 (2018).
Gagkaeva, T. Y. & Yli-Mattila, T. Emergence of Fusarium verticillioides in Finland. Eur. J. Plant Pathol. 158, 1051–1057 (2020).
West, J. S. et al. Impacts of changing climate and agronomic factors on Fusarium ear blight of wheat in the UK. Fungal Ecol. 5, 53–61 (2012).
European Commission. Green maize by area, production and humidity. Eurostat https://ec.europa.eu/eurostat/databrowser/view/tag00101/default/table?lang=en (2022).
Torp, M. & Nirenberg, H. I. Fusarium langsethiae sp. nov. on cereals in Europe. Int. J. Food Microbiol. 95, 247–256 (2004).
Góral, T., Ochodzki, P., Nielsen, L. K. & Walentyn-Góral, D. Fusarium species and Fusarium mycotoxins in grain of wheat in Poland in 2009 and 2010. Preprints 2019, 2019090108 (2019).
Lindblad, M. et al. Deoxynivalenol and other selected Fusarium toxins in Swedish wheat—occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 167, 284–291 (2013).
Infantino, A. et al. Occurrence of Fusarium langsethiae strains isolated from durum wheat in Italy. J. Phytopathol. 163, 612–619 (2015).
European Commission. EU-28: gross production by selected crops. Eurostat https://circabc.europa.eu/sd/a/826456b2-1d40-456a-80a5-3665b9c7d06a/Cereals%20Production%252c%20Area%20%26%20Yield.xlsx (2022).
Wenda-Piesik, A. et al. Fusarium head blight incidence and detection of Fusarium toxins in wheat in relation to agronomic factors. Eur. J. Plant Pathol. 149, 515–531 (2017).
Suproniene, S., Sakalauskas, S., Stumbriene, K., Zvirdauskiene, R. & Svegzda, P. Variances in trichothecene chemotype distribution in Lithuanian wheat grain and within pure culture Fusarium graminearum isolated from the same grain samples. Eur. J. Plant Pathol. 144, 371–381 (2016).
EU prices for selected representative products. European Commission https://ec.europa.eu/info/food-farming-fisheries/farming/facts-and-figures/markets/prices/price-monitoring-sector/eu-prices-selected-representative-products_en (2022).
United Nations Standard Country Codes Series M, No. 49, Revison 4 (United Nations, 1999).
Peel, M. C., Finlayson, B. L. & McMahon, T. A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 11, 1633–1644 (2007).
Acknowledgements
We would like to thank BIOMIN, the EFSA and Fera Science Limited (United Kingdom) for providing data. L.J. was funded by a University of Bath University Research Studentship Award and supported by a British Society for Plant Pathology Covid-19 PhD student support grant. D.B. was funded by Global Food Security grant no. BB/N020847/1. S.G. is a Canadian Institute for Advanced Research fellow in ‘The Fungal Kingdom: Threats and Opportunities’. N.B. was supported by the Biotechnology and Biological Sciences Research Council Future Leader Fellowship BB/N011686/1, an internal University of Bath grant, and a Royal Society grant RGS\R2\202128.
Author information
Authors and Affiliations
Contributions
L.J. contributed to the project design, performed the analyses and wrote the manuscript. D.B. and S.G. advised on the project and contributed to the preparation of the manuscript. N.B. contributed to the project’s conceptual design, funding and analyses, in addition to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Food thanks Giovanni Beccari and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Prevalence of FHB symptoms in UK wheat between 2000–2019, showing an increase in FHB incidence.
Source: DEFRA winter wheat survey.
Extended Data Fig. 2 Correlations between DON level and DON occurrence in food and feed wheat from individual European countries.
(A) DON occurrence in food and feed are strongly positively correlated, with the exclusions of Croatia, UK and Denmark (in orange). (B) DON levels in food and feed are slightly positively correlated. DON occurrence and mean DON levels are slightly negatively correlated in food (C) and positively correlated in feed (D).
Extended Data Fig. 3 Köppen–Geiger climate classification of European countries 1986–2010.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Table 1
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johns, L.E., Bebber, D.P., Gurr, S.J. et al. Emerging health threat and cost of Fusarium mycotoxins in European wheat. Nat Food 3, 1014–1019 (2022). https://doi.org/10.1038/s43016-022-00655-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43016-022-00655-z
This article is cited by
-
Temporal transcriptomics provides insights into host‒pathogen interactions: a case study of Didymella pinodella and disease-resistant and disease-susceptible pea varieties
Crop Health (2023)
-
FHB resistance conferred by Fhb1 is under inhibitory regulation of two genetic loci in wheat (Triticum aestivum L.)
Theoretical and Applied Genetics (2023)