Abstract
Pollinators support the production of the leading food crops worldwide. Organophosphates are a heavily used group of insecticides that pollinators can be exposed to, especially during crop pollination. Exposure to lethal or sublethal doses can impair fitness of wild and managed bees, risking pollination quality and food security. Here we report a low-cost, scalable in vivo detoxification strategy for organophosphate insecticides involving encapsulation of phosphotriesterase (OPT) in pollen-inspired microparticles (PIMs). We developed uniform and consumable PIMs capable of loading OPT at 90% efficiency and protecting OPT from degradation in the pH of a bee gut. Microcolonies of Bombus impatiens fed malathion-contaminated pollen patties demonstrated 100% survival when fed OPT−PIMs but 0% survival with OPT alone, or with plain sucrose within five and four days, respectively. Thus, the detrimental effects of malathion were eliminated when bees consumed OPT−PIMs. This design presents a versatile treatment that can be integrated into supplemental feeds such as pollen patties or dietary syrup for managed pollinators to reduce risk of organophosphate insecticides.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on request. Source data are provided with this paper.
References
Abernethy, V. D. Nature’s services: societal dependence on natural ecosystems. Popul. Environ. 20, 277–278 (1999).
Bartomeus, I. et al. Contribution of insect pollinators to crop yield and quality varies with agricultural intensification. PeerJ 2, e328 (2014).
Klein, A. M. et al. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313 (2007).
Kleijn, D. et al. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 6, 7414 (2015).
Reilly, J. R. et al. Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B 287, 20200922 (2020).
Croft, B. A. Arthropod Biological Control Agents and Pesticides (Wiley, 1990).
Goulson, D., Nicholls, E., Botias, C. & Rotheray, E. L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 6229 (2015).
Rissato, S. R., Galhiane, M. S., de Almeida, M. V., Gerenutti, M. & Apon, B. M. Multiresidue determination of pesticides in honey samples by gas chromatography–mass spectrometry and application in environmental contamination. Food Chem. 101, 1719–1726 (2007).
Ghini, S. et al. Occurrence and distribution of pesticides in the province of Bologna, Italy, using honeybees as bioindicators. Arch. Environ. Contam. Toxicol. 47, 479–488 (2004).
Yao, J., Zhu, Y. C., Adamczyk, J. & Luttrell, R. Influences of acephate and mixtures with other commonly used pesticides on honey bee (Apis mellifera) survival and detoxification enzyme activities. Comp. Biochem. Physiol. C 209, 9–17 (2018).
Sanchez-Bayo, F. & Goka, K. Pesticide residues and bees—a risk assessment. PLoS ONE 9, e94482 (2014).
Peter, J. V., Sudarsan, T. I. & Moran, J. L. Clinical features of organophosphate poisoning: a review of different classification systems and approaches. Indian J. Crit. Care Med. 18, 735–745 (2014).
Fukuto, T. R. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 87, 245–254 (1990).
Lerro, C. C. et al. Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the agricultural health study. Occup. Environ. Med. 72, 736–744 (2015).
Rodriguez, O. P., Muth, G. W., Berkman, C. E., Kim, K. & Thompson, C. M. Inhibition of various cholinesterases with the enantiomers of malaoxon. Bull. Environ. Contam. Toxicol. 58, 171–176 (1997).
Tomlin, C. The Insecticide Manual: A World Compendium (BCPC, 2009).
Pinjari, A. B., Pandey, J. P., Kamireddy, S. & Siddavattam, D. Expression and subcellular localization of organophosphate hydrolase in acephate-degrading Pseudomonas sp strain Ind01 and its use as a potential biocatalyst for elimination of organophosphate insecticides. Lett. Appl. Microbiol. 57, 63–68 (2013).
Zheng, Y., Lan, W., Qiao, C., Mulchandani, A. & Chen, W. Decontamination of vegetables sprayed with organophosphate pesticides by organophosphorus hydrolase and carboxylesterase. Appl. Biochem. Biotechnol. 136, 233–241 (2007).
Benning, M. M., Kuo, J. M., Raushel, F. M. & Holden, H. M. Three-dimensional structure of the binuclear metal center of phosphotriesterase. Biochemistry 34, 7973–7978 (1995).
Roodveldt, C. & Tawfik, D. S. Directed evolution of phosphotriesterase from Pseudomonas diminuta for heterologous expression in Escherichia coli results in stabilization of the metal-free state. Protein Eng. Des. Sel. 18, 51–58 (2005).
McLoughlin, S. Y., Jackson, C., Liu, J. W. & Ollis, D. Increased expression of a bacterial phosphotriesterase in Escherichia coli through directed evolution. Protein Expr. Purif. 41, 433–440 (2005).
Caldwell, S. R., Newcomb, J. R., Schlecht, K. A. & Raushel, F. M. Limits of diffusion in the hydrolysis of substrates by the phosphotriesterase from Pseudomonas diminuta. Biochemistry 30, 7438–7444 (1991).
Hong, S. B. & Raushel, F. M. Metal−substrate interactions facilitate the catalytic activity of the bacterial phosphotriesterase. Biochemistry 35, 10904–10912 (1996).
Naqvi, T. et al. A 5,000-fold increase in the specificity of a bacterial phosphotriesterase for malathion through combinatorial active site mutagenesis. PLoS ONE 9, e94177 (2014).
Benning, M. M., Kuo, J. M., Raushel, F. M. & Holden, H. M. Three-dimensional structure of phosphotriesterase: an enzyme capable of detoxifying organophosphate nerve agents. Biochemistry 33, 15001–15007 (1994).
Singh, B. K. Organophosphorus-degrading bacteria: ecology and industrial applications. Nat. Rev. Microbiol. 7, 156–164 (2009).
Sogorb, M. A., Vilanova, E. & Carrera, V. Future applications of phosphotriesterases in the prophylaxis and treatment of organophosporus insecticide and nerve agent poisonings. Toxicol. Lett. 151, 219–233 (2004).
Yang, C. Y. et al. Improved stability and half-life of fluorinated phosphotriesterase using rosetta. ChemBioChem 15, 1761–1764 (2014).
Rochu, D. et al. Contribution of the active-site metal cation to the catalytic activity and to the conformational stability of phosphotriesterase: temperature- and pH-dependence. Biochem. J. 380, 627–633 (2004).
Chen-Goodspeed, M., Sogorb, M. A., Wu, F. Y., Hong, S. B. & Raushel, F. M. Structural determinants of the substrate and stereochemical specificity of phosphotriesterase. Biochemistry 40, 1325–1331 (2001).
Jackson, C. J. et al. Structure-based rational design of a phosphotriesterase. Appl. Environ. Microbiol. 75, 5153–5156 (2009).
Pavelka, A., Chovancova, E. & Damborsky, J. Hotspot wizard: a web server for identification of hot spots in protein engineering. Nucleic Acids Res. 37, W376–W383 (2009).
Olsen, A. J. et al. Impact of phenylalanines outside the dimer interface on phosphotriesterase stability and function. Mol. Biosyst. 13, 2092–2106 (2017).
Baker, P. J. & Montclare, J. K. Enhanced refoldability and thermoactivity of fluorinated phosphotriesterase. ChemBioChem 12, 1845–1848 (2011).
Malone, L. A., Burgess, E. P. J., Stefanovic, D. & Gatehouse, H. S. Effects of four protease inhibitors on the survival of worker bumblebees, Bombus terrestris L. Apidologie 31, 25–38 (2000).
Rademacher, E., Harz, M. & Schneider, S. Effects of oxalic acid on Apis mellifera (hymenoptera: apidae). Insects 8, 84 (2017).
Bailey, L. The action of the proventriculus of the honeybee (Apis mellifera L.). Bee World 32, 92 (1951).
Peng, Y. S. & Marston, J. M. Filtering mechanism of the honey bee proventriculus. Physiol. Entomol. 11, 433–439 (1986).
Roth, R., Schoelkopf, J., Huwyler, J. & Puchkov, M. Functionalized calcium carbonate microparticles for the delivery of proteins. Eur. J. Pharm. Biopharm. 122, 96–103 (2018).
Boyjoo, Y., Pareek, V. K. & Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2, 14270–14288 (2014).
Song, J., Wang, R., Liu, Z. & Zhang, H. S. Preparation and characterization of calcium carbonate microspheres and their potential application as drug carriers. Mol. Med. Rep. 17, 8403–8408 (2018).
Fu, M. F., Wang, A. H., Zhang, X. M., Dai, L. R. & Li, J. B. Direct observation of the distribution of gelatin in calcium carbonate crystals by super-resolution fluorescence microscopy. Angew. Chem. Int. Ed. 55, 908–911 (2016).
Wszelaka-Rylik, M., Piotrowska, K. & Gierycz, P. Simulation, aggregation and thermal analysis of nanostructured calcite obtained in a controlled multiphase process. J. Therm. Anal. Calorim. 119, 1323–1338 (2015).
Sukhorukov, G. B. et al. Porous calcium carbonate microparticles as templates for encapsulation of bioactive compounds. J. Mater. Chem. 14, 2073–2081 (2004).
Griffiths, A. D. & Tawfik, D. S. Directed evolution of an extremely fast phosphotriesterase by in vitro compartmentalization. EMBO J. 22, 24–35 (2003).
Bigley, A. N. & Raushel, F. M. Catalytic mechanisms for phosphotriesterases. Biochim. Biophys. Acta 1834, 443–453 (2013).
Chen, Y. Z., Luo, Z. G. & Lu, X. X. Construction of novel enzyme−graphene oxide catalytic interface with improved enzymatic performance and its assembly mechanism. ACS Appl. Mater. Interfaces 11, 11349–11359 (2019).
Li, P. et al. Nanosizing a metal−organic framework enzyme carrier for accelerating nerve agent hydrolysis. ACS Nano 10, 9174–9182 (2016).
Wang, Y. et al. Rolling circle transcription-amplified hierarchically structured organic−inorganic hybrid RNA flowers for enzyme immobilization. ACS Appl. Mater. Interfaces 11, 22932–22940 (2019).
Som, A. et al. Monodispersed calcium carbonate nanoparticles modulate local pH and inhibit tumor growth in vivo. Nanoscale 8, 12639–12647 (2016).
Katyal, P., Chu, S. & Montclare, J. K. Enhancing organophosphate hydrolase efficacy via protein engineering and immobilization strategies. Ann. N. Y. Acad. Sci. https://doi.org/10.1111/nyas.14451 (2020).
Arena, M. & Sgolastra, F. A meta-analysis comparing the sensitivity of bees to pesticides. Ecotoxicology 23, 324–334 (2014).
Al Naggar, Y. et al. Organophosphorus insecticides in honey, pollen and bees (Apis mellifera L.) and their potential hazard to bee colonies in Egypt. Ecotoxicol. Environ. Saf. 114, 1–8 (2015).
Martin, M. M. & Lindqvist, L. The pH dependence of fluorescein fluorescence. J. Lumin. 10, 381–390 (1975).
Cochran, R. in Hayes’ Handbook of Pesticide Toxicology (ed. Krieger, R.) 337−355 (Elsevier, 2010).
Gradish, A. E. et al. Comparison of pesticide exposure in honey bees (hymenoptera: apidae) and bumble bees (hymenoptera: apidae): implications for risk assessments. Environ. Entomol. 48, 12–21 (2019).
Peng, Y. S., Nasr, M. E. & Marston, J. M. Release of alfalfa, Medicago sativa, pollen cytoplasm in the gut of the honey bee, Apis mellifera (hymenoptera: apidae). Ann. Entomol. Soc. Am. 79, 804–807 (1986).
Kroon, G., Van Praagh, J. & Velthuis, H. Osmotic shock as a prerequisite to pollen digestion in the alimentary tract of the worker honeybee. J. Apic. Res. 13, 177–181 (1974).
Chaganti, L. K., Venkatakrishnan, N. & Bose, K. An efficient method for FITC labelling of proteins using tandem affinity purification. Biosci. Rep. 38, BSR20181764 (2018).
Ellman, G. L., Courtney, K. D., Andres, V. & Featherstone, R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7, 88–95 (1961).
Acknowledgements
This material is based on work that are partially supported by the National Institute of Food and Agriculture, US Department of Agriculture, Hatch under 2017-18-107, Counter ACT Program of the National Institute of Health under Award Number R21-NS10383-01 and the National Science Foundation under Award Number IIP-1918981. This work made use of the Cornell Center for Materials Research Shared Facilities which are supported through the NSF MRSEC programme (DMR-1719875).
Author information
Authors and Affiliations
Contributions
J.C. and M.M. conceived the study. J.C. and J.W. designed and conducted the experiments. S.G. and J.-K.M. provided the E. coli strain. K.S. drew the schemes. J.C., J.W., J.-K.M., S.M. and M.M. reviewed and interpreted the results. J.C, J.W. and M.M. wrote the manuscript, with input from all authors. All authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The technology described in this paper is being licensed to Beemmunity Inc., a start-up company co-founded by J.W. M.M. and J.C. are scientific advisors and shareholders of Beemmunity Inc.
Additional information
Peer review information Nature Food thanks Liangfang Zhang, Scott Walper and Scott Medina for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7.
Source data
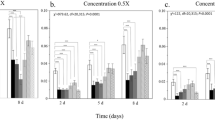
Source Data Fig. 2
Size distribution, suspension stability and pore size distribution.
Source Data Fig. 3
Protein loading efficiency and relative enzyme activity.
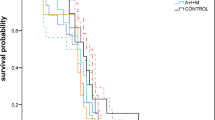
Source Data Fig. 5
Relative activity of AChE and survival data.
Rights and permissions
About this article
Cite this article
Chen, J., Webb, J., Shariati, K. et al. Pollen-inspired enzymatic microparticles to reduce organophosphate toxicity in managed pollinators. Nat Food 2, 339–347 (2021). https://doi.org/10.1038/s43016-021-00282-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43016-021-00282-0
This article is cited by
-
Monolithic-to-focal evolving biointerfaces in tissue regeneration and bioelectronics
Nature Chemical Engineering (2024)
-
Global honeybee health decline factors and potential conservation techniques
Food Security (2023)