Abstract
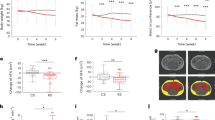
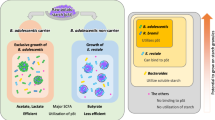
Elevated postprandial glucose (PPG) is a significant risk factor for non-communicable diseases globally. Currently, there is a limited understanding of how starch structures within a carbohydrate-rich food matrix interact with the gut luminal environment to control PPG. Here, we use pea seeds (Pisum sativum) and pea flour, derived from two near-identical pea genotypes (BC1/19RR and BC1/19rr) differing primarily in the type of starch accumulated, to explore the contribution of starch structure, food matrix and intestinal environment to PPG. Using stable isotope 13C-labelled pea seeds, coupled with synchronous gastric, duodenal and plasma sampling in vivo, we demonstrate that maintenance of cell structure and changes in starch morphology are closely related to lower glucose availability in the small intestine, resulting in acutely lower PPG and promotion of changes in the gut bacterial composition associated with long-term metabolic health improvements.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All presented data are tabulated and detailed in the main text and the Supplementary Information. The experimental procedures are detailed in the Methods. Quantified data are freely available from the Mendeley Data Database at https://doi.org/10.17632/gtthhhp9wz.1.
References
O’Keefe, J. H. & Bell, D. S. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 100, 899–904 (2007).
Wolever, T. M. & Bolognesi, C. Source and amount of carbohydrate affect postprandial glucose and insulin in normal subjects. J. Nutr. 126, 2798–2806 (1996).
Jenkins, D. et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 34, 362–366 (1981).
Jenkins, D. J. et al. Glycemic index: overview of implications in health and disease. Am. J. Clin. Nutr. 76, 266S–273S (2002).
Greenwood, D. C. et al. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: systematic review and dose–response meta-analysis of prospective studies. Diabetes Care 36, 4166–4171 (2013).
Jenkins, D. J. et al. Effect of a low–glycemic index or a high–cereal fiber diet on type 2 diabetes: a randomized trial. J. Am. Med. Assoc. 300, 2742–2753 (2008).
Wang, S. & Copeland, L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: a review. Food Funct. 4, 1564–1580 (2013).
Sievenpiper, J. et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 52, 1479 (2009).
Rayner, T. et al. Genetic variation controlling wrinkled seed phenotypes in Pisum: how lucky was Mendel? Int. J. Mol. Sci. 18, 1205 (2017).
Bhattacharyya, M. K. et al. The wrinkled-seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch-branching enzyme. Cell 60, 115–122 (1990).
Satoh, H. et al. Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm. Plant Physiol. 133, 1111–1121 (2003).
Sun, Y. et al. Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes. Front. Plant Sci. 8, 298 (2017).
Hazard, B. et al. Induced mutations in the starch branching enzyme II (SBEII) genes increase amylose and resistant starch content in durum wheat. Crop Sci. 52, 1754–1766 (2012).
Schönhofen, A., Zhang, X. & Dubcovsky, J. Combined mutations in five wheat STARCH BRANCHING ENZYME II genes improve resistant starch but affect grain yield and bread-making quality. J. Cereal Sci. 75, 165–174 (2017).
Tuncel, A. et al. Cas9‐mediated mutagenesis of potato starch‐branching enzymes generates a range of tuber starch phenotypes. Plant Biotechnol. J. 17, 2259–2271 (2019).
Ghoos, Y. F. et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 104, 1640–1647 (1993).
McCleary, B. V. & Monaghan, D. A. Measurement of resistant starch. J. AOAC Int. 85, 665–675 (2002).
Gidley, M. et al. Molecular order and structure in enzyme-resistant retrograded starch. Carbohydr. Polym. 28, 23–31 (1995).
Lopez-Rubio, A. et al. Molecular rearrangement of starch during in vitro digestion: toward a better understanding of enzyme resistant starch formation in processed starches. Biomacromolecules 9, 1951–1958 (2008).
Shrestha, A. K. et al. Enzyme resistance and structural organization in extruded high amylose maize starch. Carbohydr. Polym. 80, 699–710 (2010).
Skrabanja, V. et al. Influence of genotype and processing on the in vitro rate of starch hydrolysis and resistant starch formation in peas (Pisum sativum L.). J. Agric. Food Chem. 47, 2033–2039 (1999).
Zhou, Y., Hoover, R. & Liu, Q. Relationship between α-amylase degradation and the structure and physicochemical properties of legume starches. Carbohydr. Polym. 57, 299–317 (2004).
Lambeth, S. M. et al. Composition, diversity and abundance of gut microbiome in prediabetes and type 2 diabetes. J. Diabetes 2, 1–7 (2015).
Edwards, C. H. et al. Manipulation of starch bioaccessibility in wheat endosperm to regulate starch digestion, postprandial glycemia, insulinemia, and gut hormone responses: a randomized controlled trial in healthy ileostomy participants, 2. Am. J. Clin. Nutr. 102, 791–800 (2015).
Tahir, R. et al. Study of the structure and properties of native and hydrothermally processed wild-type, lam and r variant pea starches that affect amylolysis of these starches. Biomacromolecules 12, 123–133 (2010).
Edwards, C. H. et al. A comparison of the kinetics of in vitro starch digestion in smooth and wrinkled peas by porcine pancreatic alpha-amylase. Food Chem. 244, 386–393 (2018).
Pilichiewicz, A. N. et al. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am. J. Physiol. Endocrinol. Metab. 293, E743–E753 (2007).
Zhang, G., Ao, Z. & Hamaker, B. R. Slow digestion property of native cereal starches. Biomacromolecules 7, 3252–3258 (2006).
Donohoe, D. R. et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 13, 517–526 (2011).
Panwar, H. et al. Probiotics as potential biotherapeutics in the management of type 2 diabetes–prospects and perspectives. Diabetes Metab. Res. Rev. 29, 103–112 (2013).
Julious, S. A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 4, 287–291 (2005).
Te Morenga, L. et al. Effect of a relatively high-protein, high-fiber diet on body composition and metabolic risk factors in overweight women. Eur. J. Clin. Nutr. 64, 1323–1331 (2010).
Preston, T. & McMillan, D. Rapid sample throughput for biomedical stable isotope tracer studies. Biomed. Environ. Mass Spectrom. 16, 229–235 (1988).
Edwards, C. et al. Production of 13C labelled pea flour for use in human digestion and fermentation studies. Isot. Environ. Health Stud. 38, 139–147 (2002).
Kreymann, B., Ghatei, M. A., Williams, G. & Bloom, S. R. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 330, 1300–1304 (1987).
Garcia-Perez, I. et al. Identifying unknown metabolites using NMR-based metabolic profiling techniques. Nat. Protoc. 15, 2538–2567 (2020).
Morrison, D. J., O'Hara, J. P., King, R. F. G. J. & Preston, T. Quantitation of plasma 13C-galactose and 13C-glucose during exercise by liquid chromatography/isotope ratio mass spectrometry. Rapid Commun. Mass Spectrom. 25, 2484–2488 (2011).
Morrison, D. J. et al. A streamlined approach to the analysis of volatile fatty acids and its application to the measurement of whole‐body flux. Rapid Commun. Mass Spectrom. 18, 2593–2600 (2004).
Minekus, M. et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 5, 1113–1124 (2014).
Perez-Moral, N. et al. Ultra-high performance liquid chromatography-size exclusion chromatography (UPLC-SEC) as an efficient tool for the rapid and highly informative characterisation of biopolymers. Carbohydr. Polym. 196, 422–426 (2018).
Flanagan, B. M., Gidley, M. J. & Warren, F. J. Rapid quantification of starch molecular order through multivariate modelling of 13C CP/MAS NMR spectra. J. Chem. Commun. 51, 14856–14858 (2015).
Wang, T. L., Bogracheva, T. Y. & Hedley, C. L. Starch: as simple as A, B, C? J. Exp. Bot. 49, 481–502 (1998).
Acknowledgements
We thank E. Panteliou and L. Mendoza for their help during nasogastric and nasoduodenal tube insertion. We thank A. Sukkar and A. Cherta Murillo for their assistance in trial 4 and G. Franco Becker and C. Byrne for their help with the radioimmunoassays in trial 4. We thank E. McKay for technical assistance with the plasma [13C]glucose and urine 13C-labelled SCFA assay and S. Small for technical assistance with the breath 13C and urine 13C-labelled SCFA assay and growth of isotope-labelled pea seeds. We thank M. Parker for valuable discussions and interpretation of the microscopy images. We thank B. Hazard, QIB, for very helpful discussions of starch mutations in cereals. All clinical trials were conducted at the NIHR Imperial Clinical Research Facility; we thank all of the staff and volunteers who took part in the study. The Division of Integrative Systems Medicine and Digestive Disease at Imperial College London receives financial support from the NIHR Imperial Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London, in line with the Gut Health research theme. I.G.-P. is supported by a NIHR fellowship (NIHR-CDF-2017-10-032). C.D. gratefully acknowledges support from the Department for Environment, Food & Rural Affairs (CH0103 and CH0111, Pulse Crop Genetic Improvement Network; and LK09126) and from the Biotechnology and Biological Sciences Research Council (BBSRC; BB/L025531/1 and BBS/E/J/000PR9799). We also gratefully acknowledge the support of the BBSRC through the BBSRC Institute Strategic Programme Food Innovation and Health BB/R012512/1 and its constituent project(s) BBS/E/F/000PR10343 (Theme 1, Food Innovation) and BBS/E/F/000PR10345 (Theme 2, Digestion in the Upper GI Tract). Infrastructure support was provided by the NIHR Imperial Biochemical Research Centre and the NIHR Imperial Clinical Research Facility. G.S.F. is an NIHR Senior Investigator. This research was funded by the BBSRC (grant nos. BB/L025582/1, BB/L025418/1, BB/L025531/1 and BB/L025566/1). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
K.P. and L.J.S. should be considered as joint first authors. G.S.F. oversaw the design and implementation of the in vivo experiments. K.P. managed, assisted in the design and performed all the experimental studies in vivo, sample collection, processing and data analysis. E.S.C. designed and applied for ethics approval for the human studies. R.A. and M.K. assisted in experimental human studies 2 and 3, and M.K. also assisted in study 4. N.P. was responsible for the nasogastric and nasoduodenal tube insertion. Metabolomics analysis was performed by K.P. and I.G.-P. Metabolite identification was performed by I.G.-P. and J.I.S.-C. D.J.M. and T.P. oversaw the stable isotope analysis and data analysis, and T.P. also oversaw the labelled crop production. L.J.S. performed simulated digestions, starch analysis of pea seeds, particle size analysis of pea fragments, sample preparation for light microscopy and scanning electron microscopy, and light microscopy imaging. Preparation, sectioning and imaging of pea tissue sections were performed by R.S. and K.L.C.; K.L.C. performed scanning electron microscopy. N.P. carried out simulated digestions of flours and subsequent starch analysis; diffusion experiments using fluorescence microscopy were also performed by N.P. R.A. performed the compression experiments and M.N.C. oversaw the implementation of these experiments. T.K. and Y.Z.K. carried out solid-state NMR experiments. P.J.W., F.J.W. and C.H.E. oversaw the design and implementation of the digestions in vitro and microscopy studies. C.D. oversaw field trials of the variant pea lines and multiplication of their seeds, with quality testing for all experiments. K.P., J.A.K.M., R.C.S. and J.M.B. performed 16S rRNA gene sequencing and data analysis. G.S.F. and K.P. led the initial drafts of the manuscript. All authors contributed to the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Tables 1–14, methods and note.
Rights and permissions
About this article
Cite this article
Petropoulou, K., Salt, L.J., Edwards, C.H. et al. A natural mutation in Pisum sativum L. (pea) alters starch assembly and improves glucose homeostasis in humans. Nat Food 1, 693–704 (2020). https://doi.org/10.1038/s43016-020-00159-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43016-020-00159-8
This article is cited by
-
Innovations in functional genomics and molecular breeding of pea: exploring advances and opportunities
aBIOTECH (2024)
-
Structure–function studies of chickpea and durum wheat uncover mechanisms by which cell wall properties influence starch bioaccessibility
Nature Food (2021)
-
Give peas a chance
Nature Food (2020)