Abstract
Glycolysis is essential for the classical activation of macrophages (M1), but how glycolytic pathway metabolites engage in this process remains to be elucidated. Glycolysis leads to production of pyruvate, which can be transported into the mitochondria by the mitochondrial pyruvate carrier (MPC) followed by utilization in the tricarboxylic acid cycle. Based on studies that used the MPC inhibitor UK5099, the mitochondrial route has been considered to be of significance for M1 activation. Using genetic approaches, here we show that the MPC is dispensable for metabolic reprogramming and activation of M1 macrophages. In addition, MPC depletion in myeloid cells has no impact on inflammatory responses and macrophage polarization toward the M1 phenotype in a mouse model of endotoxemia. While UK5099 reaches maximal MPC inhibitory capacity at approximately 2–5 μM, higher concentrations are required to inhibit inflammatory cytokine production in M1 and this is independent of MPC expression. Taken together, MPC-mediated metabolism is dispensable for the classical activation of macrophages and UK5099 inhibits inflammatory responses in M1 macrophages due to effects other than MPC inhibition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (GSA) in the National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences, under accession code GSA: CRA009169 (RNA-seq data) and GSA: CRA009183 (single-cell RNA-seq data), which are publicly accessible at https://ngdc.cncb.ac.cn/gsa (refs. 49,50). Other data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
O’Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553 (2016).
Wang, A., Luan, H. H. & Medzhitov, R. An evolutionary perspective on immunometabolism. Science 363, eaar3932 (2019).
Van den Bossche, J., O’Neill, L. A. & Menon, D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 38, 395–406 (2017).
Russell, D. G., Huang, L. & VanderVen, B. C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 19, 291–304 (2019).
Ryan, D. G. & O’Neill, L. A. Krebs cycle reborn in macrophage immunometabolism. Annu. Rev. Immunol. 38, 289–313 (2020).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Mills, E. L. et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470 (2016).
Bricker, D. K. et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337, 96–100 (2012).
Herzig, S. et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science 337, 93–96 (2012).
Lauterbach, M. A. et al. Toll-like receptor signaling rewires macrophage metabolism and promotes histone acetylation via ATP-citrate lyase. Immunity 51, 997–1011 (2019).
Langston, P. K. et al. Glycerol phosphate shuttle enzyme GPD2 regulates macrophage inflammatory responses. Nat. Immunol. 20, 1186–1195 (2019).
Meiser, J. et al. Pro-inflammatory macrophages sustain pyruvate oxidation through pyruvate dehydrogenase for the synthesis of itaconate and to enable cytokine expression. J. Biol. Chem. 291, 3932–3946 (2016).
Bae, S. et al. MYC-mediated early glycolysis negatively regulates proinflammatory responses by controlling IRF4 in inflammatory macrophages. Cell Rep. 35, 109264 (2021).
Martínez-Reyes, I. et al. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol. Cell 61, 199–209 (2016).
Hill, B. G. et al. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 393, 1485–1512 (2012).
Chua, Y. L. et al. Stabilization of hypoxia-inducible factor-1α protein in hypoxia occurs independently of mitochondrial reactive oxygen species production. J. Biol. Chem. 285, 31277–31284 (2010).
Agani, F. H., Pichiule, P., Chavez, J. C. & LaManna, J. C. Inhibitors of mitochondrial complex I attenuate the accumulation of hypoxia-inducible factor-1 during hypoxia in Hep3B cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 132, 107–109 (2002).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003).
Ke, Q. & Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 70, 1469–1480 (2006).
McCommis, K. S. et al. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metab. 22, 682–694 (2015).
Shi, J., Hua, L., Harmer, D., Li, P. & Ren, G. Cre driver mice targeting macrophages. Methods Mol. Biol. 1784, 263–275 (2018).
Zhang, X., Goncalves, R. & Mosser, D. M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 83, 14–1 (2008).
Wang, F. et al. Interferon-γ induces reversible metabolic reprogramming of M1 macrophages to sustain cell viability and pro-inflammatory activity. eBioMedicine 30, 303–316 (2018).
Wang, F. et al. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metab. 28, 463–475 (2018).
Witsell, A. L. & Schook, L. B. Macrophage heterogeneity occurs through a developmental mechanism. Proc. Natl Acad. Sci. USA 88, 1963–1967 (1991).
Divakaruni, A. S., Paradyse, A., Ferrick, D. A., Murphy, A. N. & Jastroch, M. Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods Enzymol. 547, 309–354 (2014).
Vigueira, P. A. et al. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Rep. 7, 2042–2053 (2014).
Divakaruni, A. S. et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc. Natl Acad. Sci. USA 110, 5422–5427 (2013).
Divakaruni, A. S. et al. Etomoxir inhibits macrophage polarization by disrupting CoA homeostasis. Cell Metab. 28, 490–503 (2018).
Zhang, H. N. et al. Systematic identification of arsenic-binding proteins reveals that hexokinase-2 is inhibited by arsenic. Proc. Natl Acad. Sci. USA 112, 15084–15089 (2015).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Noe, J. T. et al. Lactate supports a metabolic-epigenetic link in macrophage polarization. Sci. Adv. 7, eabi8602 (2021).
Lagziel, S., Gottlieb, E. & Shlomi, T. Mind your media. Nat. Metab. 2, 1369–1372 (2020).
Gautier, E. L. et al. Gata6 regulates aspartoacylase expression in resident peritoneal macrophages and controls their survival. J. Exp. Med. 211, 1525–1531 (2014).
Rodriguez, A. E. et al. Serine metabolism supports macrophage IL-1β production. Cell Metab. 29, 1003–1011 (2019).
Baardman, J. et al. A defective pentose phosphate pathway reduces inflammatory macrophage responses during hypercholesterolemia. Cell Rep. 25, 2044–2052 (2018).
Baardman, J. et al. Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques. Nat. Commun. 11, 1–15 (2020).
Verberk, S. G. et al. Myeloid ATP citrate lyase regulates macrophage inflammatory responses in vitro without altering inflammatory disease outcomes. Front. Immunol. 12, 669920 (2021).
Wang, Y. et al. Improvement of obesity-associated disorders by a small-molecule drug targeting mitochondria of adipose tissue macrophages. Nat. Commun. 12, 1–16 (2021).
Liu, X. et al. Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell 175, 502–513 (2018).
Bensard, C. L. et al. Regulation of tumor initiation by the mitochondrial pyruvate carrier. Cell Metab. 31, 284–300 (2020).
Vacanti, N. M. et al. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol. Cell 56, 425–435 (2014).
Bender, T., Pena, G. & Martinou, J. C. Regulation of mitochondrial pyruvate uptake by alternative pyruvate carrier complexes. EMBO J. 34, 911–924 (2015).
Schell, J. C. & Rutter, J. The long and winding road to the mitochondrial pyruvate carrier. Cancer Metab. 1, 1–9 (2013).
Proudlove, M. O., Beechey, R. B. & Moore, A. L. Pyruvate transport by thermogenic-tissue mitochondria. Biochem. J. 247, 441–447 (1987).
Halestrap, A. P. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem. J. 148, 85–96 (1975).
Lee, W. N., Byerley, L. O., Bergner, E. A. & Edmond, J. Mass isotopomer analysis: theoretical and practical considerations. Biol. Mass Spectrom. 20, 451–458 (1991).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Chen, T. et al. The Genome Sequence Archive family: toward explosive data growth and diverse data types. Genom. Proteom. Bioinform. 19, 578–583 (2021).
CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 50, D27–D38 (2022).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant nos. 81970073 and 82170090 to F.W.), the Shanghai Science and Technology Commission (grant nos. 19ZR1441600 and 21Y11901100 to F.W.), the Shanghai Pujiang Program (grant no. 2020PJD051 to F.W.), the Outstanding Clinical Discipline Project of Shanghai Pudong, the Top-level Clinical Discipline Project of Shanghai Pudong (grant no. PWYgf2021-05 to Q.L.) and the Academic Leaders Training Program of Pudong Health and Family Planning Commission of Shanghai (grant no. PWRd2019-02 to F.W.).
Author information
Authors and Affiliations
Contributions
Conceptualization was the responsibility of F.W. and S.Z. Methodology was the responsibility of F.W., S.Z., R.J., J.Z., H.G. and J.H. Validation was the responsibility of F.W. and S.Z. Investigation was the responsibility of L.R., S.Z., G.W. and P.Z. Writing of the original draft was carried out by F.W. and J.H. Review and editing was carried out by F.W., S.Z., Q.L. and J.H. Supervision was the responsibility of F.W., Q.L. and J.H. Funding acquisition was the responsibility of F.W., Q.L. and J.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Jan Van den Bossche, Laurent Yvan-Charvet and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Conditional knock-out of Mpc1 in myeloid cells.
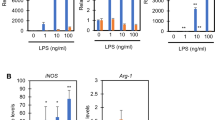
(a) Cell viability of WT BMDMs after 24 hours stimulation with LPS ± 1 hour pre-treatment with different concentrations of UK5099 (n = 6 biologically independent samples). (b) Gene targeting strategy for Mpc1 depletion in exon 3–5 in a Lyz2-conditional manner. (c) and (d) Expression of Mpc1 and Mpc2 mRNA in Mpc1fl/fl and Mpc1ΔLysM BMDMs (n = 4 biologically independent samples). (e) and (f) Expression of MPC1 and MPC2 protein in Mpc1fl/fl and Mpc1ΔLysM BMDMs. (g) and (h) Expression of Mpc1 and Mpc2 mRNA in Mpc1fl/fl and Mpc1ΔLysM AMs (n = 3 mice). (i) Cell viability of Mpc1fl/fl and Mpc1ΔLysM BMDMs after 24 hours stimulation with LPS ± 1 hour pre-treatment with 100 μM UK5099 (n = 8 biologically independent samples). Data are representative of three independent experiments (a, g, h, i). Data are representative of at least ten independent experiments (c to f). P values were calculated using one-way ANOVA with Fisher’s LSD post hoc analysis (a) or unpaired, two-sided Student’s t-test (c, d, g, h, i). Data are presented as mean ± s.e.m.
Extended Data Fig. 2 UK5099 suppresses macrophages activation independent of MPC expression in LPS-stimulated macrophages.
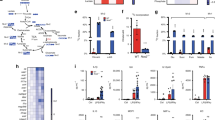
(a) Volcano plots of gene expression in Mpc1fl/fl and Mpc1ΔLysM BMDMs with or without pre-treatment with 100 μM UK5099 (Related to Figure 3g–i). (b) Venn diagrams showing overlap of UK5099 (100 μM)-altered genes between Mpc1fl/fl and Mpc1ΔLysM BMDMs with (down panel) or without (up panel) LPS stimulation. (c) Heat map of gene expression in Mpc1fl/fl and Mpc1ΔLysM BMDMs after 4 hours stimulation with LPS ± 1 hour pre-treatment with UK5099 (100 μM). The included genes were those significantly changed by UK5099 treatment in both Mpc1fl/fl and Mpc1ΔLysM BMDMs with or without LPS stimulation (Fold change˃1.5 & q-value˂0.05). (d) Flow cytometry analysis of F4/80 and CD11b expression in BMDMs. (e) UMAP clustering of scRNA-seq from Mpc1fl/fl and Mpc1ΔLysM BMDMs after 4 hours stimulation with LPS ± 1 hour pre-treatment with 100 μM UK5099. (f) UMAP plots of Adgre 1 (F4/80), Itgam (CD11b) and Lyz2 (LysM) expression among pooled samples. (g) Heat map of HIF-1α targeted gene expression of Mpc1fl/fl and Mpc1ΔLysM BMDMs after 4 hours stimulation with LPS ± 1 hour pre-treatment with 100 μM UK5099. (h) Heat map of oxidoreductase activity (GO:0016712) Gene Set in LPS-stimulated Mpc1fl/fl (left panel) and Mpc1ΔLysM (right panel) BMDMs with and without 100 μM UK5099 pre-treatment (Related to Fig. 4q). Data are representative of three independent experiments (d).
Extended Data Fig. 3 Mpc1 depletion reduces pyruvate fueling of the TCA cycle in LPS-stimulated macrophages.
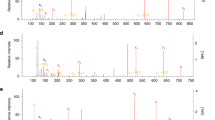
(a) to (e) U-[13C]-Pyruvate labeled TCA cycle metabolites in Mpc1fl/fl and Mpc1ΔLysM BMDMs with or without stimulation by LPS for 2 hours (n = 4 biologically independent samples). (f) to (j) U-[13C]-Pyruvate labeled TCA cycle metabolites m+3 fraction in Mpc1fl/fl and Mpc1ΔLysM BMDMs with or without stimulation by LPS for 2 hours (n = 4 biologically independent samples). Data are representative of two independent experiments (a to j). P values were calculated by two-way ANOVA with Fisher’s LSD post hoc analysis. Data are presented as mean ± s.e.m.
Extended Data Fig. 4 Acute loss of Mpc1 has no impact on proinflammatory cytokines production in LPS-stimulated macrophages.
(a) to (b) Expression of Mpc1 and Mpc2 mRNA in Mpc1fl/fl and Mpc1ΔLysM-ERT BMDMs treated with 4-Hydroxytamoxifen for 3 days (n = 3 biologically independent samples). (c) Expression of MPC1 and MPC2 protein in Mpc1fl/fl and Mpc1ΔLysM-ERT BMDMs treated with 4-Hydroxytamoxifen for 3 days. (d) to (f) Expression of proinflammatory cytokines mRNA in Mpc1fl/fl and Mpc1ΔLysM-ERT BMDMs with or without stimulation by LPS for 4 hours (n = 3 biologically independent samples). (g) to (i) Proinflammatory cytokines secretion of Mpc1fl/fl and Mpc1ΔLysM-ERT BMDMs with or without stimulation by LPS for 24 hours (n = 4 biologically independent samples). (j) to (l) Expression of proinflammatory cytokines mRNA in Mpc1fl/fl and Mpc1ΔLysM-ERT BMDMs after 4 hours stimulation with LPS ± 1 hour pre-treatment with 100 μM UK5099 (n = 3 biologically independent samples). (m) to (o) Proinflammatory cytokines secretion by Mpc1fl/fl and Mpc1ΔLysM-ERT BMDMs after 24 hours stimulation with LPS ± 1 hour pre-treatment with 100 μM UK5099 (n = 4 biologically independent samples). (p) Immunoblot analysis of histone acetylation (H3K9) in Mpc1fl/fl and Mpc1ΔLysM-ERT BMDMs with or without stimulation by LPS for the indicated times. Data are representative of at least five independent experiments (a to c, p). Data are representative of four independent experiments (d to i). Data are representative of three independent experiments (j to o). P values were calculated by two-way ANOVA with Fisher’s LSD post hoc analysis (a, b) or unpaired, two-sided Student’s t-test (d to o). Data are presented as mean ± s.e.m.
Extended Data Fig. 5 Mpc1 depletion reduces lactate fueling of the TCA cycle.
(a) to (d) U-[13C]-Lactate labeled TCA cycle metabolites in Mpc1fl/fl and Mpc1ΔLysM BMDMs (n = 5 biologically independent samples). (e) Gating strategy for peritoneal macrophages.Data are representative of two independent experiments (a to d). P values were calculated using unpaired, two-sided Student’s t-test. Data are presented as mean ± s.e.m.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed and uncropped images of western blots. RAW images of Fig. 1n.
Source Data Fig. 2
Unprocessed and uncropped images of western blots. RAW images of Fig. 2e.
Source Data Fig. 4
Unprocessed and uncropped images of western blots. RAW images of Fig. 4o.
Source Data Fig. 7
Unprocessed and uncropped images of western blots. RAW images of Fig. 7g,j,m.
Source Data Extended Data Fig. 1
Unprocessed and uncropped images of western blots. RAW images of Extended Data Fig. 1e,f.
Source Data Extended Data Fig. 4
Unprocessed and uncropped images of western blots. RAW images Extended Data Fig. 4c,p.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ran, L., Zhang, S., Wang, G. et al. Mitochondrial pyruvate carrier-mediated metabolism is dispensable for the classical activation of macrophages. Nat Metab 5, 804–820 (2023). https://doi.org/10.1038/s42255-023-00800-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00800-3
This article is cited by
-
Hypoxia promotes metastasis by relieving miR-598-3p-restricted glycolysis in gastric cancer
Journal of Translational Medicine (2024)
-
Career pathways, part 13
Nature Metabolism (2024)
-
Unraveling the complex roles of macrophages in obese adipose tissue: an overview
Frontiers of Medicine (2024)
-
Inflamed macrophages sans mitochondrial pyruvate carrier?
Nature Metabolism (2023)