Abstract
Human pluripotent stem cell-derived islets (hPSC islets) are a promising alternative to primary human islets for the treatment of insulin-deficient diabetes. We previously demonstrated the feasibility of this approach in nonhuman primates; however, the therapeutic effects of hPSC islets can be limited by the maladaptive processes at the transplantation site. Here, we demonstrate successful implantation of hPSC-derived islets in a new transplantation site in the abdomen, the subanterior rectus sheath, in eight nonhuman primates (five male and three female). In this proof-of-principle study, we find that hPSC islets survive and gradually mature after transplantation, leading to improved glycemic control in diabetic primates. Notably, C-peptide secretion responds to meal challenge from 6 weeks post-transplantation (wpt), with stimulation indices comparable to those of native islets. The average post-prandial C-peptide level reaches approximately 2.0 ng ml−1 from 8 wpt, which is five times higher than the peak value we previously obtained after portal vein infusion of hPSC islets and was associated with a decrease of glycated hemoglobin levels by 44% at 12 wpt. Although additional studies in larger cohorts involving long-term follow-up of transplants are needed, our results indicate that the subanterior rectus sheath supports functional maturation and maintenance of hPSC islets, suggesting that it warrants further exploration as a transplantation target site in the context of for hPSC-based cell-replacement therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Spatial transcriptomic sequencing data have been deposited in the CNGB Nucleotide Sequence Archive of China National GeneBank DataBase with accession number CNP0003618 (https://db.cngb.org/search/project/CNP0003618/). Other experimental data, materials or reagents are available upon request and will be released subject to a material transfer agreement. Source data are provided with this paper.
Change history
16 January 2023
In the version of the article initially published, there was a unit error in Figure 3a–d,i–l, where “ng dl−1” appeared rather than “ng ml−1”, as now appears in the corrected HTML and PDF versions of the article.
References
Du, Y. et al. Human pluripotent stem-cell-derived islets ameliorate diabetes in non-human primates. Nat. Med. 28, 272–282 (2022).
Pagliuca, F. W. et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014).
Rezania, A. et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32, 1121–1133 (2014).
Balboa, D. et al. Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01219-z (2022).
Grapin-Botton, A. & Ludwig, B. Stem cell-derived β cells go in monkeys. Cell Stem Cell 29, 500–502 (2022).
Rickels, M. R. & Robertson, R. P. Pancreatic Islet transplantation in humans: recent progress and future directions. Endocr. Rev. 40, 631–668 (2019).
McCall, M. & Shapiro, A. M. Update on islet transplantation. Cold Spring Harb. Perspect. Med 2, a007823 (2012).
Brusko, T. M., Russ, H. A. & Stabler, C. L. Strategies for durable β cell replacement in type 1 diabetes. Science 373, 516–522 (2021).
Shapiro, A. M. et al. The portal immunosuppressive storm: relevance to islet transplantation? Therapeutic Drug Monit. 27, 35–37 (2005).
Cantarelli, E. & Piemonti, L. Alternative transplantation sites for pancreatic islet grafts. Curr. Diab. Rep. 11, 364–374 (2011).
Cunha, G. R. & Baskin, L. Use of sub-renal capsule transplantation in developmental biology. Differ. Res. Biol. Diversity 91, 4–9 (2016).
Buckingham, M. Skeletal muscle formation in vertebrates. Curr. Opin. Genet. Dev. 11, 440–448 (2001).
Wigmore, P. M. & Evans, D. J. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis. Int. Rev. Cytol. 216, 175–232 (2002).
Sevensma, K.E., Leavitt, L. & Pihl, K.D. Anatomy, Abdomen and Pelvis, Rectus Sheath. (StatPearls Publishing, 2022).
Yu, M. et al. Islet transplantation in the subcutaneous space achieves long-term euglycaemia in preclinical models of type 1 diabetes. Nat. Metab. 2, 1013–1020 (2020).
Bertuzzi, F., Colussi, G., Lauterio, A. & De Carlis, L. Intramuscular islet allotransplantation in type 1 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 22, 1731–1736 (2018).
Sakata, N. et al. Strategy for clinical setting in intramuscular and subcutaneous islet transplantation. Diabetes/Metab. Res. Rev. 30, 1–10 (2014).
Rafael, E. et al. Intramuscular autotransplantation of pancreatic islets in a 7-year-old child: a 2-year follow-up. Am. J. Transplant. 8, 458–462 (2008).
Guan, J. et al. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature 605, 325–331 (2022).
Blum, B. et al. Functional β-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat. Biotechnol. 30, 261–264 (2012).
Kaneto, H. et al. PDX-1 and MafA play a crucial role in pancreatic β-cell differentiation and maintenance of mature β-cell function. Endocr. J. 55, 235–252 (2008).
Zhu, H., Yu, L., He, Y. & Wang, B. Nonhuman primate models of type 1 diabetes mellitus for islet transplantation. J. Diabetes Res. 2014, 785948 (2014).
Shin, J. S. et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am. J. Transplant. 15, 2837–2850 (2015).
Sherwani, S. I., Khan, H. A., Ekhzaimy, A., Masood, A. & Sakharkar, M. K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights 11, 95–104 (2016).
American Diabetes, A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes 2021. Diabetes Care 44, S15–S33 (2021).
Bottino, R. et al. Recovery of endogenous β-cell function in nonhuman primates after chemical diabetes induction and islet transplantation. Diabetes 58, 442–447 (2009).
Shapiro, A. M. et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 355, 1318–1330 (2006).
Leighton, E., Sainsbury, C. A. & Jones, G. C. A practical review of C-peptide testing in diabetes. Diabetes Ther. 8, 475–487 (2017).
Jones, A. G. & Hattersley, A. T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. 30, 803–817 (2013).
Matsumoto, S. & Shimoda, M. Current situation of clinical islet transplantation from allogeneic toward xenogeneic. J. Diabetes 12, 733–741 (2020).
Graham, M. L., Bellin, M. D., Papas, K. K., Hering, B. J. & Schuurman, H. J. Species incompatibilities in the pig-to-macaque islet xenotransplant model affect transplant outcome: a comparison with allotransplantation. Xenotransplantation 18, 328–342 (2011).
Kim, J. M. et al. Induction, management, and complications of streptozotocin-induced diabetes mellitus in rhesus monkeys. Xenotransplantation 23, 472–478 (2016).
Graham, M. L. et al. Refining the high-dose streptozotocin-induced diabetic non-human primate model: an evaluation of risk factors and outcomes. Exp. Biol. Med. 236, 1218–1230 (2011).
Silver, B. et al. EADSG guidelines: insulin therapy in diabetes. Diabetes Ther. 9, 449–492 (2018).
Chen, A. et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell 185, 1777–1792 (2022).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Acknowledgements
This work was supported by National Natural Science Foundation of China (32288102 to H.D.); National Science and Technology Innovation 2030 Major Program (2021ZD0200900 to X.P.); CAMS Innovation Fund for Medical Sciences (CIFMS,2021-I2M-1-024 to X.P.); Key R&D Program of Zhejiang (2022C03SA170260 to Y.D.). We thank J. Lou for the discussion and advice on the transplantation site for human islets. Q. Yao at the Department of Clinical Pharmacology of the First Affiliated Hospital of Kunming Medical University for therapeutic drug monitoring; B. Xie at the Key Laboratory of Organ Development and Regeneration of Zhejiang Province for confocal image acquisition. We thank J. Vaughan at the Salk Institute for Biological Studies and M. Huising at the University of California, Davis for their kind provision of the UCN3 antibody. We thank C. Wang for animal research ethics guidance and J. Cao for clinical assistance with the macaques. We thank X. Yuan for his original drawings in Extended Data Fig. 1. We thank B. Liu, L. Wang, J Guan, Y. Fu and L. Cheng for discussions in the course of the preparation of this manuscript. We thank all authors of our previous nonhuman primate study1, which laid the foundation for this study.
Author information
Authors and Affiliations
Contributions
H.D., Y.D. and X.P. supervised the project. H.D. and Y.D. conceived the experimental design. Y.D., S.S., S.L. and D.S. wrote the manuscript. Z.L., D.S., S.L., Z. L. and W.Y. performed most of the experiments on nonhuman primates. S.W. and Y.W. generated hPSC islets for transplantation. Z.L., Y.W., Y.Z. and S.L. recovered and qualified cells for transplantation. Z.L., J.Y., Y.S., Z.W., X.F., W.S. and H.L. performed the characterization of hPSC-islet grafts. S.W. and Z.S. designed the treatment plan of diabetic recipients. W.S. and Z.L. gave advice on the transplantation site for hPSC islets. Y.L. performed routine blood and biochemical tests.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Gordon Weir and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
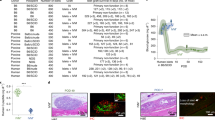
Extended Data Fig. 1 Schematic of hPSC-islet transplantation strategies.
a, hPSC islets were transplanted into three extraperitoneal sites for comparison. b, Under the guidance of ultrasound imaging, hPSC islets were transplanted into the subanterior rectus sheath using a puncture needle. c, hPSC-islet was delivered by eight injections, with four parallel injections on each side of the abdominis rectus.
Extended Data Fig. 2 Immunostaining with human cell marker and islet enriched transcription factors in hPSC-islet grafts in three extraperitoneal transplantation sites at 1 week post-transplantation.
a, Representative immunohistochemical staining of human cell marker Stem121 in human grafts in Monkey 1 and 2, which results showed the morphology and structure of residual human grafts in three tested transplantation sites at 1 wpt. Scale bars, 400 μm (Monkey 1) and 200 μm (Monkey 2); for the enlarged view (indicated with green border), 50 μm (Monkey 1) and 25 μm (Monkey 2). Similar results were obtained from five independent grafts. b-c, Representative immunofluorescence staining of C-peptide and β cell specific transcription factor PDX1 (b) or NKX2.2 (c) in human grafts in Monkey 1. Scale bar, 50 μm. Similar results were obtained from five independent grafts. d, Quantitative assessment of the expression of islet-enriched transcription factors (PDX1, NKX6.1 and NKX2.2) in human grafts in three indicated sites (n = 5 independent grafts). Data presented as mean ± SEM. Two-tailed t-test was used to determine significance between groups and P value was indicated above the bars.
Extended Data Fig. 3 Evaluation of hPSC-islet grafts in Monkey 3 and 4 at 4 weeks post-transplantation.
a, Representative immunohistochemical staining of human cell marker Stem121 in three indicated graft sites, which results showed the morphology and structure of residual human grafts in three tested transplantation sites at 4 wpt. Scale bars, 400 μm; for the enlarged view (indicated with green border), 50 μm. Similar results were obtained from five independent grafts. b, Representative immunofluorescence staining of islet hormones in subanterior rectus sheath grafts. Scale bar, 50 μm. Similar results were obtained from five independent grafts. c, Representative immunofluorescence staining of pancreatic transcription factors (TFs) and UCN3 in subanterior rectus sheath grafts in Monkey 3. Scale bar, 50 μm. Similar results were obtained from five independent grafts. d, Representative immunohistochemical staining of endothelial marker CD31 in subanterior rectus sheath grafts. Scale bars, 100 μm; for the enlarged view (indicated with green border), 25 μm. Similar results were obtained from five independent grafts.
Extended Data Fig. 4 Evaluation of hPSC-islet grafts in Monkey 3 and 4 at 4 weeks post-transplantation.
a, Representative immunofluorescence staining of pancreatic transcription factors (TFs) and UCN3 in subanterior rectus sheath grafts in Monkey 4. Scale bar, 50 μm. Similar results were obtained from five independent grafts. b, Representative immunohistochemical staining of T cell marker CD3, B cell marker CD20 and macrophage marker CD68 in subanterior rectus sheath grafts (left). Monkey spleen or liver sections were used as control. The quantitative analysis of immunological infiltration in hPSC-islet grafts (n = 10 independent grafts) (right). Data presented as mean ± SEM. Scale bars, 100 μm.
Extended Data Fig. 5 Subanterior rectus sheath transplantation of hPSC islets improved glycemic control in immunosuppressed diabetic rhesus macaques.
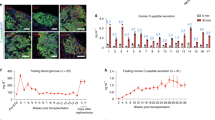
a-d, Daily fasting blood glucose levels of diabetic macaque recipients before STZ treatment (gray), before (brown) and after (black) subanterior rectus sheath transplantation of hPSC islets (infusion procedure conducted at day 0). e-h, Blood glucose levels of diabetic monkeys at 1 month before transplantation (Pre-Tx) and 3 months after sub-rectus sheath transplantation (9 - 12 wpt) (n = 28 independent values). i, C-peptide secretion levels of four diabetic monkeys (Monkey 5 - 8) before transplantation (Pre-Tx), 6 weeks (6 wpt) and 3 months (3 mpt) after sub-rectus sheath transplantation (n = 4 monkeys). The C-peptide secretion level of diabetic monkeys at 3 months after intraportal infusion of hPSC islets (Du et al., 3 mpt) was cited for comparison (n = 4 monkeys). Data presented as mean ± SEM. Two-tailed t-test was used to determine significance between groups and P value was indicated above the bars.
Extended Data Fig. 6 Characterization of subanterior rectus sheath hPSC-islet grafts in Monkey 6-8 at 13 wpt.
a-f, Representative immunofluorescence staining of islet hormones, pancreatic transcription factors (PDX1, NKX6.1, NKX2.2 and MAFA) and UCN3 in subanterior rectus sheath grafts. Scale bar, 50 μm. Similar results were obtained from five independent grafts. g, Representative immunohistochemical staining of endothelial marker CD31 in subanterior rectus sheath grafts. Scale bars, 100 μm; for the enlarged view (indicated with green border), 25 μm. Similar results were obtained from five independent grafts.
Extended Data Fig. 7 The in situ gene expression analysis of hPSC-islet grafts under anterior rectus sheath in Monkey 5 and 6 using Stereo-seq.
a-b, Hematoxylin and eosin (H&E) staining and immunohistochemical staining of human cell marker Stem121 indicated the human grafts in rectus abdominis tissues of Monkey 5 (a) and 6 (b). Scale bars, 400 μm. c-f, The expression of islet-enriched hormones and transcription factors (c and d) and functional genes (e and f) in rectus abdominis sections of Monkey 5 (c and e) and 6 (d and f). OXPHOS, oxidative phosphorylation. The results of spatial transcriptomics analysis were reproduced on two independent recipient macaques. Similar human grafts (Stem121 positive) could be obtained from five independent sections.
Extended Data Fig. 8 Characterization of hPSC-islet grafts of Monkey 5 and 6 at 12 weeks after sub-rectus sheath transplantation.
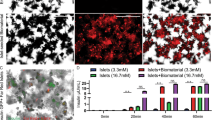
a-b, Representative immunofluorescence staining of PCSK1 (a) and PCSK2 (b) in subanterior rectus sheath grafts and human islets. Scale bar, 50 μm. Similar results were obtained from five independent grafts. c, Vessel density in hPSC-islet grafts and native human islets (n = 10 independent grafts). Two-tailed t-test was used to determine significance between groups and P value was indicated above the bars. d, Representative immunohistochemical staining of T cell marker CD3, B cell marker CD20 and macrophage marker CD68 in subanterior rectus sheath grafts (left). Monkey spleen or liver sections were used as control. The quantitative analysis of immunological infiltration in hPSC-islet grafts (n = 10 independent grafts) (right). Scale bars, 100 μm. Data presented as mean ± SEM. Two-tailed t-test was used without adjustments for multiple comparisons to determine significance between groups and P value was indicated above the bars.
Extended Data Fig. 9 Gross anatomy, histological and immunological analysis of native pancreas of STZ-treated recipient monkeys.
a, Gross anatomy and H&E staining of pancreas sections of recipient monkeys, with islet structures outlined in white. Scale bar, 100 μm. Similar results were obtained from five independent sections. b, C-peptide staining of pancreas sections of STZ-treated recipient monkeys. Scale bars, 200 μm; for the enlarged view (indicated with green border), 50 μm. Similar results were obtained from five independent sections. c-e, Representative immunofluorescence staining of CHGA (c), islet hormones (d) and CK19 and Proinsulin (e) in pancreas sections of STZ-treated recipient Monkeys. Scale bar, 50 μm. Similar results were obtained from five independent sections.
Extended Data Fig. 10 Ultrasound examination and postmortem examination of major organs in transplanted diabetic macaques.
Gross anatomy (a, d, g and j), H&E staining (b, e, h and k) and ultrasound examination (c, f, i and l) of major organs of Monkey 5 - 8. Scale bar, 200 μm. n.a., not available. Similar results of H&E staining were obtained from five independent sections.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11 and Supplementary Tables 1–10.
Supplementary Video 1
The procedure of subanterior rectus sheath transplantation under the guidance of ultrasound.
Supplementary Data 1
Statistical Source Data for Supplementary Fig. 1
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Z., Sun, D., Lu, S. et al. Implantation underneath the abdominal anterior rectus sheath enables effective and functional engraftment of stem-cell-derived islets. Nat Metab 5, 29–40 (2023). https://doi.org/10.1038/s42255-022-00713-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00713-7
This article is cited by
-
From stem cells to pancreatic β-cells: strategies, applications, and potential treatments for diabetes
Molecular and Cellular Biochemistry (2024)
-
Estimating residual undifferentiated cells in human chemically induced pluripotent stem cell derived islets using lncRNA as biomarkers
Scientific Reports (2023)