Abstract
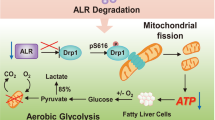
Oxidative stress modulates carcinogenesis in the liver; however, direct evidence for metabolic control of oxidative stress during pathogenesis, particularly, of progression from cirrhosis to hepatocellular carcinoma (HCC), has been lacking. Deficiency of transaldolase (TAL), a rate-limiting enzyme of the non-oxidative branch of the pentose phosphate pathway (PPP), restricts growth and predisposes to cirrhosis and HCC in mice and humans. Here, we show that mitochondrial oxidative stress and progression from cirrhosis to HCC and acetaminophen-induced liver necrosis are critically dependent on NADPH depletion and polyol buildup by aldose reductase (AR), while this enzyme protects from carbon trapping in the PPP and growth restriction in TAL deficiency. Both TAL and AR are confined to the cytosol; however, their inactivation distorts mitochondrial redox homeostasis in opposite directions. The results suggest that AR acts as a rheostat of carbon recycling and NADPH output of the PPP with broad implications for disease progression from cirrhosis to HCC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All original data will be available through sharing and databases with accession codes provided by the time of publication. RNA-seq data have been deposited in the NCBI Gene Expression Omnibus database (accession code GSE217133). Source data are provided with this paper.
Change history
08 February 2023
A Correction to this paper has been published: https://doi.org/10.1038/s42255-023-00752-8
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Perl, A., Hanczko, R., Telarico, T., Oaks, Z. & Landas, S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol. Med. 7, 395–403 (2011).
Nelson, M.E. et al. Inhibition of hepatic lipogenesis enhances liver tumorigenesis by increasing antioxidant defence and promoting cell survival. Nat. Commun. https://doi.org/10.1038/ncomms14689 (2017).
Choi, J. E. et al. A unique subset of glycolytic tumour-propagating cells drives squamous cell carcinoma. Nat. Metab. 3, 182–195 (2021).
Sullivan, L. et al. The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol. Cell 51, 236–248 (2013).
Horecker, B. L. The pentose phosphate pathway. J. Biol. Chem. 277, 47965–47971 (2002).
Li, M. et al. Aldolase B suppresses hepatocellular carcinogenesis by inhibiting G6PD and pentose phosphate pathways. Nat. Cancer 1, 735–747 (2020).
Vulliamy, T., Mason, P. & Luzzatto, L. The molecular basis of glucose-6-phosphate dehydrogenase deficiency. Trends Genet. 8, 138–143 (1992).
Pandolfi, P. P., Sonati, F., Rivi, R., Mason, P. & Grosveld, F. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 14, 5209–5215 (1995).
Perl, A. et al. Transaldolase is essential for maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa. Proc. Natl Acad. Sci. USA 103, 14813–14818 (2006).
Hanczko, R. et al. Prevention of hepatocarcinogenesis and acetaminophen-induced liver failure in transaldolase-deficient mice by N-acetylcysteine. J. Clin. Invest. 119, 1546–1557 (2009).
LeDuc, C. et al. Novel association of early onset hepatocellular carcinoma with transaldolase deficiency. JIMD Rep. 12, 121–127 (2014).
Grammatikopoulos, T. et al. Liver disease and risk of hepatocellular carcinoma in children with mutations in TALDO1. Hepatol. Commun. https://doi.org/10.1002/hep4.1824 (2021).
Williams, M. et al. Clinical, biochemical, and molecular overview of transaldolase deficiency and evaluation of the endocrine function: update of 34 patients. J. Inher. Metab. Dis. 42, 147–158 (2019).
Qian, Y. et al. Transaldolase deficiency influences the pentose phosphate pathway, mitochondrial homoeostasis and apoptosis signal processing. Biochem. J. 415, 123–134 (2008).
Oaks, Z. et al. Transaldolase haploinsufficiency in subjects with acetaminophen-induced liver failure. J. Inherit. Metab. Dis. 43, 496–506 (2020).
Jones, B. E., Liu, H., Lo, C. R., Koop, D. R. & Czaja, M. J. Cytochrome P450 2E1 expression induces hepatocyte resistance to cell death from oxidative stress. Antiox. Redox Signal. 4, 701–709 (2002).
Dahlin, D. C., Miwa, G. T., Lu, A. Y. & Nelson, S. D. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc. Natl Acad. Sci. USA 81, 1327–1331 (1984).
Vas, G. Y. et al. Study of transaldolase deficiency in urine samples by capillary LC–MS/MS. J. Mass. Spec. 41, 463–469 (2005).
Wilmarth, P. A. et al. A systems approach implicates nuclear receptor targeting in the Atp7b-/- mouse model of Wilson’s disease. Metallomics 4, 660–668 (2012).
Nordgaard, C. et al. ZAKβ is activated by cellular compression and mediates contraction-induced MAP kinase signaling in skeletal muscle. EMBO J. https://doi.org/10.15252/embj.2022111650 (2022).
Lee, K. W., Ko, B. C., Jiang, Z., Cao, D. & Chung, S. S. Overexpression of aldose reductase in liver cancers may contribute to drug resistance. Anticancer Drugs 12, 129–132 (2001).
Rodan, L. H. & Berry, G. T. N-acetylcysteine therapy in an infant with transaldolase deficiency is well tolerated and associated with normalization of α fetoprotein levels. JIMD Rep. 31, 73–77 (2016).
El-Serag, H.B. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology https://doi.org/10.1053/j.gastro.2004.09.013 (2004).
Koutselini, H., Lazaris, A. C., Kavantzas, N., Kiritsi, T. & Davaris, P. S. Significance of nuclear morphometry as a diagnostic tool in fine-needle aspirates of the liver. Eur. J. Gastroenterol. Hepatol. 12, 913–921 (2000).
Lee-Barber, J. et al. Apparent acetaminophen toxicity in a patient with transaldolase deficiency. JIMD Rep. 44, 9–15 (2019).
Lee, W. M. Acetaminophen and the US Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology 40, 6–9 (2004).
Jang, C., Chen, L. & Rabinowitz, J. D. Metabolomics and isotope tracing. Cell 173, 822–837 (2018).
Oaks, Z. et al. Mitochondrial dysfunction in the liver and antiphospholipid antibody production precede disease onset and respond to rapamycin in lupus-prone mice. Arthritis Rheumatol. 68, 2728–2739 (2016).
Kim, T. W. et al. NQO1 deficiency leads enhanced autophagy in cisplatin-induced acute kidney injury through the AMPK/TSC2/mTOR signaling pathway. Antioxid. Redox Signal. 24, 867–883 (2016).
Koch, B. et al. Crystal structures of glutaminyl cyclases (QCs) from Drosophila melanogaster reveal active site conservation between insect and mammalian QCs. Biochemistry 51, 7383–7392 (2012).
Wellner, V. P., Sekura, R., Meister, A. & Larsson, A. Glutathione synthetase deficiency, an inborn error of metabolism involving the g-glutamyl cycle in patients with 5-oxoprolinuria (pyroglutamic aciduria). Proc. Natl Acad. Sci. USA 71, 2505–2509 (1974).
Zhong, H. et al. Mitochondrial control of apoptosis through modulation of cardiolipin oxidation in hepatocellular carcinoma: a novel link between oxidative stress and cancer. Free Rad. Biol. Med. 102, 67–76 (2017).
Shi, F. et al. Oxidative damage of DNA, RNA and their metabolites in leukocytes, plasma and urine of Macaca mulatta: 8-oxoguanosine in urine is a useful marker for aging. Free Rad. Res. 46, 1093–1098 (2012).
Soga, T. et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 281, 16768–16776 (2006).
Gao, N. et al. Hyperhomocysteinemia-Induced oxidative stress aggravates renal damage in hypertensive rats. Am. J. Hypertens. 33, 1127–1135 (2020).
Hayashi, M. et al. High serum uric acid levels could be a risk factor of hepatocellular carcinoma recurrences. Nutr. Cancer 73, 996–1003 (2021).
Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009).
Kadenbach, B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim. Biophys. Acta 1604, 77–94 (2003).
De la Fuente, I. M. et al. On the dynamics of the adenylate energy system: homeorhesis vs homeostasis. PLoS ONE 9, e108676 (2014).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Zhang, J. et al. 13C isotope-assisted methods for quantifying glutamine metabolism in cancer cells. Meth. Enzymol. 542, 369–389 (2014).
Engelke, U. F. H. et al. Mitochondrial involvement and erythronic acid as a novel biomarker in transaldolase deficiency. Biochim. Biophys. Acta 1802, 1028–1035 (2010).
Kotas, M. E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015).
Sohn, M.-J. et al. Metabolomic signatures for the effects of weight loss interventions on severe obesity in children and adolescents. Metabolites https://doi.org/10.3390/metabo12010027 (2022).
Giustarini, D. et al. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic. Biol. Med. 112, 360–375 (2017).
Mueller, C. F. H. et al. The role of the multidrug resistance protein-1 in modulation of endothelial cell oxidative stress. Circ. Res. 97, 637 (2005).
Ishikawa, T. & Sies, H. Cardiac transport of glutathione disulfide and S-conjugate. Studies with isolated perfused rat heart during hydroperoxide metabolism. J. Biol. Chem. 259, 3838–3843 (1984).
Lovat, P. E. et al. Bak: a downstream mediator of fenretinide-induced apoptosis of SH-SY5Y neuroblastoma cells. Cancer Res. 63, 7310–7313 (2003).
Tarrio, N., Garcia-Leiro, A., Cerdan, M. E. & Gonzalez-Siso, M. I. The role of glutathione reductase in the interplay between oxidative stress response and turnover of cytosolic NADPH in Kluyveromyces lactis. FEMS Yeast Res. 8, 597–606 (2008).
Racker, E. & Schroeder, E. Formation and utilization of octulose-8-phosphate by transaldolase and transketolase. Arch. Biochem. Biophys. 66, 241–243 (1957).
Paoletti, F., Williams, J. F. & Horecker, B. L. Detection and estimation of sedoheptulose and octulose mono- and bisphosphates in extracts of rat liver. Arch. Biochem. Biophys. 198, 620–626 (1979).
Stincone, A. et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 90, 927–963 (2015).
Kovarova, J. et al. Deletion of transketolase triggers a stringent metabolic response in promastigotes and loss of virulence in amastigotes of Leishmania mexicana. PLoS Pathog. 14, e1006953 (2018).
Fukumoto, S. I. et al. Overexpression of the aldo-keto reductase family protein AKR1B10 is highly correlated with smokers’ non-small cell lung carcinomas. Clin. Cancer Res. 11, 1776 (2005).
Ma, J. et al. AKR1B10 overexpression in breast cancer: Association with tumor size, lymph node metastasis and patient survival and its potential as a novel serum marker. Int. J. Cancer 131, E862–E871 (2012).
Ho, H. T. et al. Aldose reductase-deficient mice develop nephrogenic diabetes insipidus. Mol. Cell. Biol. 20, 5840–5846 (2000).
Edmondson, H. A. & Steiner, P. E. Primary carcinoma of the liver. A study of 100 cases among 48,900 necropsies. Cancer 7, 462–503 (1954).
International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the International Consensus Group for Hepatocellular Neoplasia. Hepatology https://doi.org/10.1002/hep.22709 (2009).
Yuan, M., Breitkopf, S. B., Yang, X. & Asara, J. M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Prot. 7, 872–881 (2012).
Xia, J., Broadhurst, D. I., Wilson, M. & Wishart, D. S. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics 9, 280–299 (2013).
Barski, O. A., Gabbay, K. H., Grimshaw, C. E. & Bohren, K. M. Mechanism of human aldehyde reductase: characterization of the active site pocket. Biochemistry 34, 11261–11275 (1995).
Banki, K., Halladay, D. & Perl, A. Cloning and expression of the human gene for transaldolase: a novel highly repetitive element constitutes an integral part of the coding sequence. J. Biol. Chem. 269, 2847–2851 (1994).
Acknowledgements
This work was supported in part by grants RO1 DK078922 (A. Perl), R01 AI072648 (A. Perl), P30CA006516 (J.M.A.) and P01CA120964 (J.M.A.) from the National Institutes of Health and the Central New York Community Foundation (A. Perl).
Author information
Authors and Affiliations
Contributions
A. Perl conceived and designed the study. A. Perl, Z.O., A. Patel, N.H., J.A., T.F. and D.K. developed methodology, executed experiments and analyzed data. M.B., S.B., J.L., M.D. and R.K. acquired the data (bred, genotyped and provided animals matched for age and sex). D.R.F., T.W. and G.C. designed, executed and analyzed experiments. S.L. and K.B. designed experiments and analyzed data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Gregory Ducker, Huiyong Yin and Christian Frezza for their contribution to the peer review of this work. Editor recognition statement Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
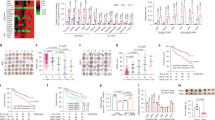
Extended Data Fig. 1 Impact of AR on carbon recycling and NADPH output by PPP.
a, Bar chart shows opposite effects of AR inactivation on concentrations of substrates sequestered in the non-oxidative branch of the PPP with respect to those depleted in the oxidative PPP and connected pathways. Data represent individual and mean values from five mice per genotype. Changes in metabolite concentration affected by TAL deficiency at two-tailed t-test p < 0.05 are shown. b, Global impact of TAL deficiency on the metabolome is indicated by analysis of pathways arranged according to the scores from enrichment analysis (y axis: −log p) and from topology analysis using betweenness centrality to estimate node importance (x axis: impact: number of detected metabolites with significant p value, using two-tailed t-test via Metaboanalyst). TAL deficiency most prominently impacted the PPP, glycerophospholipid and glycine/serine/threonine (G/S/T), valine/leucine/isoleucine (V/L/I) and alanine, aspartate, glutamate (A/D/E) amino acid metabolism. c, Global impact of AR deficiency on metabolic pathways, using two-tailed t-test via Metaboanalyst Pathway analysis module. d, Global impact of combined TAL and AR deficiency on metabolic pathways by comparison of DKO and WT mice, using two-tailed t-test via Metaboanalyst Pathway analysis module. e, Western blot detection recombinant AR (rAR, left panel) and assays of its enzyme activity (right panel). Data represent independent measurements for the following substrates: glyceraldehyde (GAD, n = 28), ascorbate (n = 2), E4P (n = 3), S7P (n = 4), OAA (n = 7), PGA (n = 8), erythrose (n = 8), sedoheptulose (n = 18). f, Schematic diagram of metabolic pathways affected by inactivation of the TAL-AR axis.
Extended Data Fig. 2 Segregation of metabolites by impact of AR inactivation in TAL deficiency.
a, Metabolites affected synergistically by inactivation of the TAL-AR axis. b, Metabolite changes corrected by inactivation of AR. Displayed metabolites exhibited significant fold changes in TAL deficiency at two-tailed t-test p < 0.05.
Extended Data Fig. 3 Western blot analysis of expression in genes implicated by RNA-seq changes in livers of TAL-deficient mice at FDR p < 0.05.
a, Western blot analysis of protein levels corrected by inactivation of AR. *, two-tailed t-test p < 0.05. b, Western blot analysis of protein levels uncorrected by inactivation of AR. Representative blots and bar charts of cumulative analysis of five mice per genotype are shown for each gene. *, two-tailed t-test p < 0.05.
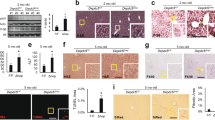
Extended Data Fig. 4 Effect of the TAL-AR axis on de novo GSH biosynthesis.
a, Measurement of GSH synthesis intermediates in liver of WT (n = 4), TALKO (n = 4), ARKO (n = 5), and DKO mice (n = 5). *, p < 0.05 relative to WT using two-tailed t-test. Brackets indicate differences between mouse strains at p < 0.05. b, Enrichment of [M1-13C]-PGA, [M2-13C]-PGA, and [M5-13C]-GSH in hepatocytes of WT (n = 4), TALKO (n = 4), ARKO (n = 4), and DKO mice (n = 4) labeled with [U-13C]-glutamine (DLM-1150-0.5, Cambridge Isotope Laboratories; Cambridge, MA) for 24 hours. *, p < 0.05 relative to WT using one-tailed t-test. c, Schematic diagram of GSH biosynthesis involving substrates regulated by the TAL-AR axis.
Extended Data Fig. 5 Effect of aldose reductase inhibitors, zopolrestat and sorbinil, on serum replenishment-induced proliferation of HepG2 hepatoma and MCF7 breast carcinoma cells.
MCF7 or HepG2 cells seeded 96-well plates at 5,000 cells/well in complete Dulbecco’s minimal essential medium with 10% fetal bovine serum (Hyclone, Logan, UT), 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 25 µg/ml amphotericin B (GIBCO/Thermo Fisher catalog number 15240096) at 37 °C with 5% CO2. After 24 hours, sub-confluent cells were growth arrested in 0.1% FBS with or without sorbinil (Millipore/Sigma catalog number S7701) or zopolrestat (Millipore/Sigma catalog number Z4527). After 24 h, 10% serum was added to the medium and the cells were incubated for another 24 h. Cells were counted after trypan blue staining (upper and lower left panels and upper right panel) and viability was evaluated by the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay (lower right panel)1. MTT powder was dissolved in PBS to a final concentration of 5 mg/ml, and 500 µl of MTT solution was added to cells and incubated for 1 h at 37oC. Subsequently, 500 µl of isopropyl alcohol with 0.04 N HCl was added to dissolve the precipitate. Absorbance was read at the 570-nm wavelength subtracting background reading at the 650-nm wavelength. Data represent four experiments. Brackets reflect p values <0.05 as compared by 1-way ANOVA.
Extended Data Fig. 6 Effect of siRNA-mediated knockdown of IDH2 on metabolic flux through the TCA cycle in primary hepatocytes from age-matched female WT, TALKO, ARKO, and DKO mice.
a, Effect of IDH2 knockdown on enrichment of [M4-13C]-citrate in hepatocytes labelled with [U-13C]-glutamine (DLM-1150-0.5, Cambridge Isotope Laboratories; Cambridge, MA) for 24 hours. b, Effect of IDH2 knockdown on enrichment of [M1-13C]-citrate, and [M2-13C]-citrate in hepatocytes labelled with [1, 2-13C]-glucose (CLM-504-0.5, Cambridge Isotope Laboratories; Cambridge, MA) for 24 hours. Data represent mean ± SE of experiments using 4 WT, 4 TALKO, 4 ARKO, and 4 DKO mice. *, Brackets reflect p values 0.05 using unpaired two-tailed t-tests for comparison of mice with different genotypes. Effect of IDH2 knockdown was evaluated with two-tailed paired t-tests; p values <0.05 are displayed.
Extended Data Fig. 7 IDH2 moderates carbon sequestration in the non-oxidative PPP in primary DKO hepatocytes.
a, Effect of IDH2 knockdown on enrichment of [M1-13C]-S7P and [M2-13C]-S7P in WT, TALKO, ARKO, and DKO hepatocytes labelled with [1, 2-13C]-glucose (CLM-504-0.5, Cambridge Isotope Laboratories; Cambridge, MA) for 24 hours. b, Effect of IDH2 knockdown on enrichment of [M5-13C]-O8P and [M6-13C]-O8P in WT, TALKO, ARKO, and DKO hepatocytes labelled with [1, 2-13C]-glucose (CLM-504-0.5, Cambridge Isotope Laboratories; Cambridge, MA) for 24 hours. c, Effect of IDH2 knockdown on enrichment of [M1-13C]-R5P and [M2-13C]-R5P and [M1-13C]-G6P and [M2-13C]-G6P in WT, TALKO, ARKO, and DKO hepatocytes labelled with [1, 2-13C]-glucose (CLM-504-0.5, Cambridge Isotope Laboratories; Cambridge, MA) for 24 hours. d, Effect of IDH2 knockdown on enrichment of [M2-13C]-erythronic acid in WT, TALKO, ARKO, and DKO hepatocytes labelled with [1, 2-13C]-glucose (CLM-504-0.5, Cambridge Isotope Laboratories; Cambridge, MA) for 24 hours. Data represent mean ± SE of experiments using 4 WT, 3 TALKO, 4 ARKO, and 4 DKO mice. *, p < 0.05 relative to WT using unpaired two-tailed t-tests. Brackets reflect p values 0.05 using 2-way ANOVA.
Supplementary information
Supplementary Information
List of abbreviations (pages 2–4); legends for Supplementary Figs. 1–8 (pages 4–5); Supplementary References (page 5); Supplementary Figs. 1–8 (pages 6–13); Supplementary Table 1 (pages 14–20) and Supplementary Table 2 (pages 21–32).
Supplementary Data
Source Data for Supplementary Figs. 1–8.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oaks, Z., Patel, A., Huang, N. et al. Cytosolic aldose metabolism contributes to progression from cirrhosis to hepatocarcinogenesis. Nat Metab 5, 41–60 (2023). https://doi.org/10.1038/s42255-022-00711-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00711-9
This article is cited by
-
ZMIZ2 facilitates hepatocellular carcinoma progression via LEF1 mediated activation of Wnt/β-catenin pathway
Experimental Hematology & Oncology (2024)
-
Rab4A-directed endosome traffic shapes pro-inflammatory mitochondrial metabolism in T cells via mitophagy, CD98 expression, and kynurenine-sensitive mTOR activation
Nature Communications (2024)
-
CATase-immobilized hydrogel platform molded by photo-enzyme coupling polymerization for effectively preventing postoperative abdominal adhesion
Science China Chemistry (2023)