Abstract
Cysteine dioxygenase 1 (Cdo1) is a key enzyme in taurine synthesis. Here we show that Cdo1 promotes lipolysis in adipose tissue. Adipose-specific knockout of Cdo1 in mice impairs energy expenditure, cold tolerance and lipolysis, exacerbates diet-induced obesity (DIO) and decreases adipose expression of the key lipolytic genes encoding ATGL and HSL, with little effect on adipose taurine levels. White-adipose-specific overexpression of ATGL and HSL blunts the role of adipose Cdo1 deficiency in promoting DIO. Mechanistically, Cdo1 interacts with PPARγ and facilitates the recruitment of Med24, the core subunit of mediator complex, to ATGL and HSL gene promoters, thereby transactivating their expression. Further, mice with transgenic overexpression of Cdo1 show better cold tolerance, ameliorated DIO and higher lipolysis capacity. Thus, we uncover an unexpected and important role of Cdo1 in regulating adipose lipolysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw RNA-seq data used to generate Figure 4a–c are available at the GEO datasets (accession number: GSE207548). The data used to generate the main results shown in the main figures and extended figures are available as source data. Source data including uncropped western blots are provided with this paper. All data supporting the findings of this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Zobel, E. H., Hansen, T. W., Rossing, P. & von Scholten, B. J. Global changes in food supply and the obesity epidemic. Curr. Obes. Rep. 5, 449–455 (2016).
Luan, X. et al. Exercise as a prescription for patients with various diseases. J. Sport Health Sci. 8, 422–441 (2019).
Luo, H.-Y., Zhu, J.-Y., Chen, M., Mu, W.-J. & Guo, L. Krüppel-like factor 10 (KLF10) as a critical signaling mediator: Versatile functions in physiological and pathophysiological processes. Genes Dis. (in the press).
Canale, M. P. et al. Obesity-related metabolic syndrome: mechanisms of sympathetic overactivity. Int J. Endocrinol. 2013, 865965 (2013).
Li, B. Y., Guo, Y. Y., Xiao, G., Guo, L. & Tang, Q. Q. SERPINA3C ameliorates adipose tissue inflammation through the Cathepsin G/Integrin/AKT pathway. Mol. Metab. 61, 101500 (2022).
Zhu, J.-Y., Chen, M., Mu, W.-J., Luo, H.-Y. & Guo, L. The functional role of Higd1a in mitochondrial homeostasis and in multiple disease processes. Genes Dis. (in the press).
Klaus, S. Functional differentiation of white and brown adipocytes. Bioessays 19, 215–223 (1997).
Lichtenbelt, W. D. V. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Mu, W. J., Zhu, J. Y., Chen, M. & Guo, L. Exercise-mediated browning of white adipose tissue: its significance, mechanism and effectiveness. Int. J. Mol. Sci. 22, 11512 (2021).
Lafontan, M. & Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 48, 275–297 (2009).
Zechner, R. et al. Fat signals–lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15, 279–291 (2012).
Haemmerle, G. et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312, 734–737 (2006).
Ahmadian, M. et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 13, 739–748 (2011).
Ahmadian, M. et al. Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes 58, 855–866 (2009).
Kim, K. et al. Adipocyte PHLPP2 inhibition prevents obesity-induced fatty liver. Nat. Commun. 12, 1822 (2021).
Festuccia, W. T., Laplante, M., Berthiaume, M., Gelinas, Y. & Deshaies, Y. PPARγ agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia 49, 2427–2436 (2006).
Kershaw, E. E. et al. PPARγ regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am. J. Physiol. Endocrinol. Metab. 293, E1736–E1745 (2007).
McCoy, J. G. et al. Structure and mechanism of mouse cysteine dioxygenase. Proc. Natl Acad. Sci. USA 103, 3084–3089 (2006).
Chen, M., Zhu, J.-Y., Mu, W.-J. & Guo, L. Cysteine dioxygenase type 1 (CDO1): Its functional role in physiological and pathophysiological processes. Genes Dis. (in the press).
Tsuboyama-Kasaoka, N. et al. Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology 147, 3276–3284 (2006).
Niewiadomski, J. et al. Effects of a block in cysteine catabolism on energy balance and fat metabolism in mice. Ann. N. Y. Acad. Sci. 1363, 99–115 (2016).
Ueki, I. et al. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am. J. Physiol. Endocrinol. Metab. 301, E668–E684 (2011).
Deng, P. et al. Cysteine dioxygenase type 1 promotes adipogenesis via interaction with peroxisome proliferator-activated receptor gamma. Biochem. Biophys. Res. Commun. 458, 123–127 (2015).
Zhao, X. et al. Cysteine dioxygenase type 1 inhibits osteogenesis by regulating Wnt signaling in primary mouse bone marrow stromal cells. Sci. Rep. 6, 19296 (2016).
Madsen, M. S., Siersbaek, R., Boergesen, M., Nielsen, R. & Mandrup, S. Peroxisome proliferator-activated receptor gamma and C/EBPα synergistically activate key metabolic adipocyte genes by assisted loading. Mol. Cell. Biol. 34, 939–954 (2014).
Wang, W. & Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17, 691–702 (2016).
Guo, Y. Y., Li, B. Y., Peng, W. Q., Guo, L. & Tang, Q. Q. Taurine-mediated browning of white adipose tissue is involved in its anti-obesity effect in mice. J. Biol. Chem. 294, 15014–15024 (2019).
Ide, T., Kushiro, M., Takahashi, Y., Shinohara, K. & Cha, S. mRNA expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism 51, 1191–1197 (2002).
Baliou, S. et al. Significance of taurine transporter (TauT) in homeostasis and its layers of regulation (Review). Mol. Med. Rep. 22, 2163–2173 (2020).
Dominy, J. E. Jr. et al. Synthesis of amino acid cofactor in cysteine dioxygenase is regulated by substrate and represents a novel post-translational regulation of activity. J. Biol. Chem. 283, 12188–12201 (2008).
Conaway, R. C., Sato, S., Tomomori-Sato, C., Yao, T. & Conaway, J. W. The mammalian mediator complex and its role in transcriptional regulation. Trends Biochem. Sci. 30, 250–255 (2005).
Ge, K. et al. Alternative mechanisms by which mediator subunit MED1/TRAP220 regulates peroxisome proliferator-activated receptor gamma-stimulated adipogenesis and target gene expression. Mol. Cell. Biol. 28, 1081–1091 (2008).
Kuang, J. Y. et al. Fat-specific Sirt6 ablation sensitizes mice to high-fat diet-induced obesity and insulin resistance by inhibiting lipolysis. Diabetes 66, 1159–1171 (2017).
Li, J. et al. Adipose HuR protects against diet-induced obesity and insulin resistance. Nat. Commun. 10, 2375 (2019).
Yu, S. et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) overexpression. J. Biol. Chem. 278, 498–505 (2003).
Kim, J. Y., Tillison, K., Lee, J. H., Rearick, D. A. & Smas, C. M. The adipose tissue triglyceride lipase ATGL/PNPLA2 is downregulated by insulin and TNF-α in 3T3-L1 adipocytes and is a target for transactivation by PPARγ. Am. J. Physiol. Endocrinol. Metab. 291, E115–E127 (2006).
McTernan, P. G. et al. Insulin and rosiglitazone regulation of lipolysis and lipogenesis in human adipose tissue in vitro. Diabetes 51, 1493–1498 (2002).
Tamori, Y., Masugi, J., Nishino, N. & Kasuga, M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes 51, 2045–2055 (2002).
Ge, K. et al. Transcription coactivator TRAP220 is required for PPARγ2-stimulated adipogenesis. Nature 417, 563–567 (2002).
Chen, S. et al. Systemic nanoparticle-mediated delivery of pantetheinase vanin-1 regulates lipolysis and adiposity in abdominal white adipose tissue. Adv. Sci. 7, 2000542 (2020).
Sluse, F. E. et al. Mitochondrial UCPs: new insights into regulation and impact. Biochem. Biophys. Acta 1757, 480–485 (2006).
Barbera, M. J. et al. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J. Biol. Chem. 276, 1486–1493 (2001).
Meex, R. C. et al. ATGL-mediated triglyceride turnover and the regulation of mitochondrial capacity in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 308, E960–E970 (2015).
Haemmerle, G. et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 17, 1076–U1082 (2011).
Jimenez, V. et al. In vivo adeno-associated viral vector-mediated genetic engineering of white and brown adipose tissue in adult mice. Diabetes 62, 4012–4022 (2013).
Zhang, Z. et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 5, 5493 (2014).
Soukas, A., Socci, N. D., Saatkamp, B. D., Novelli, S. & Friedman, J. M. Distinct transcriptional profiles of adipogenesis in vivo and in vitro. J. Biol. Chem. 276, 34167–34174 (2001).
Pan, D., Fujimoto, M., Lopes, A. & Wang, Y. X. Twist-1 is a PPAR∆-inducible, negative-feedback regulator of PGC-1α in brown fat metabolism. Cell 137, 73–86 (2009).
Guo, L. et al. Enhanced acetylation of ATP-citrate lyase promotes the progression of nonalcoholic fatty liver disease. J. Biol. Chem. 294, 11805–11816 (2019).
Guo, L., Guo, Y. Y., Li, B. Y., Peng, W. Q. & Tang, Q. Q. Histone demethylase KDM5A is transactivated by the transcription factor C/EBP and promotes preadipocyte differentiation by inhibiting Wnt/-catenin signaling. J. Biol. Chem. 294, 9642–9654 (2019).
Liu, Y. et al. Kruppel-like factor 10 (KLF10) is transactivated by the transcription factor C/EBP and involved in early 3T3-L1 preadipocyte differentiation. J. Biol. Chem. 293, 14012–14021 (2018).
Virtue, S., Lelliott, C. J. & Vidal-Puig, A. What is the most appropriate covariate in ANCOVA when analysing metabolic rate? Nat. Metab. 3, 1585–1585 (2021).
Muller, T. D., Klingenspor, M. & Tschop, M. H. Revisiting energy expenditure: how to correct mouse metabolic rate for body mass. Nat. Metab. 3, 1134–1136 (2021).
Virtue, S. & Vidal-Puig, A. GTTs and ITTs in mice: simple tests, complex answers. Nat. Metab. 3, 883–886 (2021).
Peng, W. Q. et al. l-Theanine activates the browning of white adipose tissue through the AMPK/α-ketoglutarate/Prdm16 axis and ameliorates diet-induced obesity in mice. Diabetes 70, 1458–1472 (2021).
Zhu, J. Y., Chen, M., Mu, W. J., Luo, H. Y. & Guo, L. Higd1a facilitates exercise-mediated alleviation of fatty liver in diet-induced obese mice. Metabolism 134, 155241 (2022).
Guo, L. et al. Acetylation of mitochondrial trifunctional protein α-Subunit enhances its stability to promote fatty acid oxidation and is decreased in nonalcoholic fatty liver disease. Mol. Cell. Biol. 36, 2553–2567 (2016).
Guo, L. et al. Hepatic neuregulin 4 signaling defines an endocrine checkpoint for steatosis-to-NASH progression. J. Clin. Invest. 127, 4449–4461 (2017).
Acknowledgements
This study was supported by the National Natural Science Foundation (NSFC) grants (81730021 and 32070760 to Q.-Q. T. and 32070751 and 31871435 to L. G.) and National Key R&D Program of China (2018YFA0800401) to Q.-Q. T, and was funded by Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai. We thank S.-W. Qian (School of Basic Medical Sciences, Fudan University) for providing AD-Cre virus.
Author information
Authors and Affiliations
Contributions
Y.-Y. G. and L. G. were involved in study design, conceived the idea, conducted the experiments, analyzed the data and drafted the paper; B.-Y. L., G. X. and Y. L. performed the experiments; L. G. and Q.-Q. T. designed and supervised the study, obtained the funding and cowrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Matthew Watt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Isabella Samuelson, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
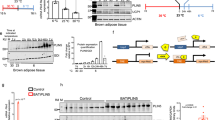
Extended Data Fig. 1 Cdo1 expression profiles and generation of Cdo1AKO mice.
a: Relative UCP1 protein fold change in iWAT before or after cold exposure in Fig. 1h (n = 3 mice). b: Relative UCP1 protein fold change in BAT before or after cold exposure in Fig. 1h (n = 3 mice). c: The mRNA levels of Cdo1 at different time points (days after adipgenic induction) during the adipogenic differentiation of DE2-3 brown adipocytes (n = 6 biological replicates). d: The representative western blot for the expression of the indicated proteins at different time points of during the adipogenic differentiation of DE2-3 brown adipocytes. e: The relative protein fold change of Cdo1 and UCP1 in d (n = 3 biological replicates). From f-j, the Cdo1AKO mice (Cdo1flox/folx/Adiponectin-Cre+) were generated by cross-breeding Cdo1flox/flox and adiponectin promoter driven Cre transgenic mice. Cdo1flox/flox /Adiponectin-Cre− mice were used as wild-type control (WT). f: Schema of Cdo1flox/folx/Adiponectin-Cre+ mouse model. g: DNA electrophoretogram for analyzing the genotype of control (Ctrl) mice (Cdo1Ctrl/Ctrl), Cdo1flox/Ctrl, Cdo1flox/flox mice. h: The mRNA levels of Cdo1 in the indicated tissues from WT and Cdo1AKO mice (n = 6 mice). i: Representative western blot of Cdo1 and HSP90 in the indicated tissues from WT and Cdo1AKO mice. j: The relative Cdo1 protein fold change in i (n = 4 mice). For statistical analysis, one-way analysis of variance plus Bonferroni’s post hoc tests were performed in c and e. Unpaired two-tailed Student’s tests were performed in a, b, h and j. All data show the means ± SD.
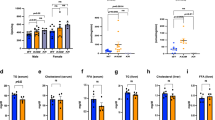
Extended Data Fig. 2 Cdo1AKO impairs lipolysis in adipose tissue of lean mice.
Mice were treated as Fig. 2. From a-d, metabolic cage experiments were performed in the male 8-week-old Cdo1AKO or Cdo1flox/flox mice (WT). Mice were fed with NCD. a: Carbon dioxide emission (VCO2) (a) and regression-based analysis of absolute VCO2 emission against body weight (b) of the mice measured in a metabolic cage of WT (n = 5 mice) and Cdo1AKO mice (n = 6 mice). c-d: Respiratory exchange ratio (RER) (c) and food intake (d) of WT (n = 5 mice) and Cdo1AKO mice (n = 6 mice). e-l: After 18 h of fasting, cold tolerance test in the fasted state was performed in the mice and then the mice were sacrificed for analysis after 6 h cold exposure. e: Western blots in BAT of WT mice and Cdo1AKO mice (n = 3 mice). HSP90-1 is the loading control for Cdo1, ATGL, HSL, UCP1 and p-ATGL. HSP90-2 is the loading control for p-HSL. f: Relative protein fold change in e (n = 3 mice). g: Western blots in eWAT of WT mice and Cdo1AKO mice (n = 3 mice). HSP90-1 is the loading control for ATGL and HSL; HSP90-2 is the loading control for p-ATGL and p-HSL. h: Relative protein fold change in g (n = 3 mice). i: The free fatty acid (FFA) levels of BAT in WT mice and Cdo1AKO mice (n = 6 mice). j: Representative immunoblot in livers of WT and Cdo1AKO mice. k: Relative protein fold change in j (n = 3 mice). l: The mRNA level of Taut in iWAT and BAT (n = 6 mice). For statistical analysis, unpaired two-tailed Student’s tests were performed in c, d, f, h, i, k and l. Two-sided ANCOVA analysis was performed in b. Two-way analysis of variance was performed in a. All data show the means ± SD. Data were compared to WT group.
Extended Data Fig. 3 Cdo1AKO impairs EE and insulin sensitivity in HFD-fed mice.
Mice were treated as indicated in Fig. 3. a: Quantification of adipocyte diameter of BAT in Fig. 3e (n = 6 mice). b-i, Metabolic cage data in the Cdo1AKO or WT mice which were fed on HFD for 4 weeks, n = 7 mice. b: Oxygen consumption rates (VO2) of WT and Cdo1AKO mice, n = 7 mice. c: Regression-based analysis of absolute VO2 against body weight, n = 7 mice. d: Heat production of WT and Cdo1AKO mice, n = 7 mice. e: Regression-based analysis of absolute heat production against body weight, n = 7 mice. f: Carbon dioxide emission (VCO2) of WT and Cdo1AKO mice, n = 7 mice. g: Regression-based analysis of absolute (VCO2) against body weight, n = 7 mice. h:Respiratory Exchange Ratio (RER) in WT and Cdo1AKO mice (n = 7 mice). i: Food intake of the mice (n = 7 mice). j-k: Representative western blot analysis for AKT and its phosphorylation form (p-AKT, s473) (j) and gray scale of p-AKT/AKT (k) in iWAT and liver of WT and Cdo1AKO mice fed with HFD for 2 weeks (n = 3 mice). HSP90-1 is the loading control for p-AKT; HSP90-2 is the loading control for AKT. Mice were administered with insulin for 20 min, after which they were sacrificed and the tissues were harvested for analysis. For statistical analysis, two-way analysis of variance was perfoed in b, d and f. Two-sided ANCOVA analysis was performed in c, e and g. Unpaired two-tailed Student’s tests were performed in a, h, i and k. All data show the means ± SD. Data were compared between WT group and Cdo1AKO group.
Extended Data Fig. 4 Cdo1AKO inhibits lipolysis and browning in adipocytes.
a-g, Mice were treated as indicated in Fig. 3. The 8-week-old WT and Cdo1AKO mice were fed on HFD for 15 weeks, and then the mice were sacrificed for analysis after 18 h of fasting. a: The mRNA level of the indicated genes in iWAT from WT and Cdo1AKO mice (n = 7 mice). b: Oxygen consumption of iWAT (n = 7 mice). c: Western blots in BAT (n = 3 mice). HSP90-1 is the loading control for Cdo1, UCP1 and ATGL; HSP90-2 is the loading control for HSL, p-HSL and p-ATGL. d: Relative protein fold change in c (n = 3 mice). e: Oxygen consumption of BAT (n = 7 mice). f: Western blots in eWAT (n = 3 mice). HSP90-1 is the loading control for ATGL and HSL; HSP90-2 is the loading control for p-ATGL and p-HSL. g: Relative protein fold change in f (n = 3 mice). h-i, SVFs were isolated from iWAT of 8-week-old male Cdo1flox/flox mice and differentiated to primary adipocytes. Then cells were treated with adenovirus harboring Cre (for knocking out Cdo1) or LacZ (for control). h: The mRNA level of the indicated genes in primary adipocytes which was treated with AD-Cre (for Cdo1 knockout) or AD-LacZ (for control) (n = 6 biological replicates). AD means adenovirus. i: Oxygen consumption in primary adipocytes (n = 5 biological replicates). For statistical analysis, unpaired two-tailed Student’s tests were performed in a, b, d, e, g, h and i. All data show the means ± SD. Data were compared to WT group in a, b, d, e, g, and compared to AD-LacZ group in h and i.
Extended Data Fig. 5 Cdo1 promotes lipolysis in in adipocytes.
a-b: The glycerol (a) and FFA (b) release in human adipocytes with or without Cdo1 knockdown under basal or ISO (10 μM)-stimulated conditions (n = 6 biological replicates). c-d: The glycerol (c) and FFA (d) release in human adipocytes, which were infected with the adenoviruses of AD-Cdo1, AD-Y157F or AD-LacZ (control) under basal or ISO (10 μM)-stimulated conditions (n = 6 biological replicates). From e-j: Experiments were performed in C3H10T1/2 adipocytes. e: The mRNA level of the indicated genes (n = 6 biological replicates). f: Representative immunoblot. HSP90-1 is the loading control for ATGL, HSL and Cdo1; HSP90-2 is the loading control for p-ATGL and p-HSL. g: Relative protein fold change in f (n = 3 biological replicates). h-i: The glycerol (h) and FFA (i) release under basal or CL316,243 (1 μM)-stimulated conditions (n = 6 biological replicates). j: Oxygen consumption in C3H10T1/2 adipocytes (n = 6 biological replicates). k: C3H10T1/2 adipocytes were infected with AD-Cdo1 or AD-LacZ, then the cells were treated with 10 μM GW6471 (PPARα antagonist) for 24 h before being harvested. The mRNA levels of the indicated genes were analyzed (n = 6 biological replicates). l: The mRNA level of the indicated genes in C3H10T1/2 adipocytes which were treated with taurine (n = 6 biological replicates). From m-o, C3H10T1/2 adipocytes were infected with AD-Cdo1 or AD-LacZ (for control). After 24 h, cells were transfected with siNC or two separated siRNA against ATGL (siATGL-1, siATGL-2). After 24 h of siRNA infection, cells were harvested. m: Oxygen consumption (n = 6 biological replicates). n: Representative immunoblot. o: Relative protein fold change in n (n = 3 biological replicates). For statistical analysis, one-way ANOVAs were performed in a, b, c, d, e, g, h, i, j, k, l, m and o. All data show the means ± SD. Data were compared to the control groups, or as indicated.
Extended Data Fig. 6 Lipolysis is the main driver of Cdo1AKO-mediated phenotypes.
Male mice were treated in Fig. 5 and mice were sacrificed for analysis after 12-week HFD feeding. From a-d, the 6-week-male mice were injected with AAV-ATGL/HSL (for overexpressing ATGL and HSL in fat pads), or AAV-GFP (control) in eWAT and iWAT. Two weeks later, mice were subjected to HFD feeding. Fourteen weeks after the AAV administration, mice were sacrificed for analysis. a: Examination of AAV-mediated overexpression of ATGL (AAV-ATGL) in various tissues by using RT-qPCR (n = 6 mice). b: Examination of AAV-HSL overexpression in various tissues by using RT-qPCR (n = 6 mice). c: Western blot of ATGL and HSL and their phosphorylation levels in iWAT and eWAT (n = 3 mice). HSP90-1 is the loading control for ATGL and HSL; HSP90-2 is the loading control for p-ATGL and p-HSL. d: Relative protein fold change in c (n = 3 mice). e: The mRNA levels of the indicated genes in iWAT (n = 6 mice). f: The mRNA levels of the indicated genes in BAT (n = 6 mice). g: Hepatic triglycerides (TAG) levels in mice (n = 6 mice). h: The mRNA levels of lipogenesis-related genes and inflammation-related genes in mice livers (n = 6 mice). For statistical analysis, unpaired two-tailed Student’s tests were performed in a, b, d and e-h. All data show the means ± SD of at least three biological replicates.
Extended Data Fig. 7 Cdo1 interacts with PPARγ and Med24 to transactivate HSL.
a: Immunofluorescence of Flag-Cdo1 in C3H10T1/2 adipocytes (n = 3 biological replicates). b: Immunoblot of Flag-Cdo1 or Flag-Y157F in C3H10T1/2 adipocytes. c: Relative exogenous Cdo1 protein level in b (n = 3 biological replicates). d: The HSL promoter (−2100 to 1 bp from the transcription start site) luciferase reporter analysis in HEK293T cells (n = 6 biological replicates). Data were compared to Vector group, or as indicated. e: Immunofluorescence of Flag-Y157F and PPARγ in C3H10T1/2 adipocytes (n = 3 biological replicates). f: Flag-Cdo1 enrichment on the indicated promoters in C3H10T1/2 adipocytes (n = 6 biological replicates). g: The PPARγ knockdown efficiency in C3H10T1/2 adipocytes (n = 6 biological replicates). h: The effect of Med24 knockdown on the indicated genes expression in C3H10T1/2 adipocytes (n = 6 biological replicates). i-j: Relative protein fold change in Fig. 6k for IP (i) and input (j) (n = 3 biological replicates). IP means immunoprecipitation. k-l: Relative protein fold change in Fig. 6l for endogenous IP (k) and input (l) in primary adipocytes (n = 3 biological replicates). m-n: Relative protein fold change in Fig. 6l for endogenous IP (m) and input (n) in eWAT (n = 3 biological replicates). o: Relative protein fold change of input in Fig. 6m (n = 3 biological replicates). p: Enrichment of PPARγ or Med24 on HSL promoterin primary adipocytes (n = 6 biological replicates). q: Relative protein fold change of input in Fig. 6p (n = 3 biological replicates). r: The enrichment of PPARγ or Med24 on HSL promoter of HSL in C3H10T1/2 adipocytes (n = 6 biological replicates). For statistical analysis, one-way ANOVAs were performed in d, f, g, h, q and r. Unpaired two-tailed Student’s tests were performed in i, k, m, o and p. Data were compared with siNC group in g and h, and compared with AD-LacZ group in q and r. All data show the means ± SD.
Extended Data Fig. 8 Cdo1TG promotes adaptive thermogenesis and iWAT browning.
a: Schema of fat specific Cdo1 transgenic (Cdo1TG) mouse model. b: The mRNA levels of Cdo1 in control (WT) and Cdo1TG mice adipose tissue (n = 6 mice). c: Representative western blot of 3*Flag tagged Cdo1 in adipose tissues. d: Relative 3*Flag tagged Cdo1 protein fold change in c (n = 3 mice). e: Representative western blot of both endogenous and exogenous Cdo1 in adipose tissues. f: Relative endogenous and exogenous Cdo1 protein fold change in e (n = 3 mice). From g-l, the 8-week-old WT and Cdo1TG male mice were fed with NCD. After 18 h of fasting, cold tolerance test and infrared thermal imaging were performed and then the mice were sacrificed (6 h after cold exposure) for analysis. g: Rectal temperature of the NCD-fed mice exposed to cold (4°C) in fasted state (n = 6 mice). h: Representative infrared thermal imaging of NCD-fed mice exposed to cold (4 °C) for 4 h (n = 3 mice). i: Photographs of iWAT from WT and Cdo1TG mice after 6 h cold exposure. j: Protein levels of lipolysis associated protein and UCP1 in iWAT from WT and Cdo1TG mice after 6 h of cold exposure (n = 3 mice). HSP90-1 is the loading control for ATGL, HSL and UCP1; HSP90-2 is the loading control for p-ATGL and p-HSL. k: Relative protein fold change in j (n = 3 mice). l: The mRNA level of the gene encoding Taut in iWAT and BAT from control and Cdo1TG mice (n = 6 mice). For statistical analysis, unpaired two-tailed Student’s tests were performed in b, d, f, k and l. Two-way analysis of variance were performed in g. All data show the means ± SD.
Extended Data Fig. 9 A graph model of adipose Cdo1-mediated lipolysis.
In adipose tissue, the expression level of adipose Cdo1 was increased by cold exposure and fasting and decreased in HFD-fed mice. In adipocytes, Cdo1 is mainly localized in cytosol and is also localized in nuclei. In the nuclei of mature adipocytes, Cdo1 is co-localized with PPARγ, and it can promote the interaction of PPARγ and Med24 (the important component of mediator complex), and then facilitates the recruitment of Med24 to ATGL and HSL gene promoters to transactivate the expression of ATGL and HSL. FFA, the products of lipolysis, could act as a ligand of PPARα to activate it. And then PPARα increases the expression of fatty acid oxidation (FAO) genes (such as Cpt1b) and browning genes (such as Ucp1). In the absence of Cdo1, less Med24 can be recruited to ATGL and HSL gene promoters, while overexpressing Cdo1 facilitates the binding of Med24 to ATGL and HSL gene promoters. Further studies are needed to investigate whether Cdo1 can also facilitate the recruitment of other mediator subunits to ATGL and HSL gene promoters. In the way above, Cdo1 facilitates PPARγ-mediated transactivation of key lipolytic genes expression to promote lipolysis, FAO and thermogenic programing in adipocytes, thereby ameliorating DIO and related metabolic disorders in mice. It should be noted that male mice were used in this paper to study the effect of manipulating adipose Cdo1 expression on the metabolic homeostasis of adipose tissue and the development of DIO and related metabolic disorders. Further studies are needed to investigate whether adipose Cdo1 also has protective role against DIO and the negative consequences of obesity in female mice. The graph model was created with biorender.com.
Supplementary information
Supplementary Information
Supplementary gels for repeated western blot data, Supplementary images for Figures and Extended Data Figures and Supplementary Tables 1–4
Source data
Source Data Fig. 1
Statistical source data
Source Data Fig. 1
Unprocessed western blots
Source Data Fig. 2
Statistical source data
Source Data Fig. 2
Unprocessed western blots
Source Data Fig. 3
Statistical source data
Source Data Fig. 4
Statistical source data
Source Data Fig. 4
Unprocessed western blots
Source Data Fig. 5
Unprocessed statistical source data
Source Data Fig. 6
Statistical source data
Source Data Fig. 6
Unprocessed gels
Source Data Fig. 7
Statistical source data
Source Data Fig. 7
Unprocessed gels
Source Data Extended Data Fig. 1
Statistical source data
Source Data Extended Data Fig. 1
Unprocessed gels
Source Data Extended Data Fig. 2
Statistical source data
Source Data Extended Data Fig. 2
Unprocessed gels
Source Data Extended Data Fig. 3
Statistical source data
Source Data Extended Data Fig. 3
Unprocessed gels
Source Data Extended Data Fig. 4
Statistical source data
Source Data Extended Data Fig. 4
Unprocessed gels
Source Data Extended Data Fig. 5
Statistical source data
Source Data Extended Data Fig. 5
Unprocessed gels
Source Data Extended Data Fig. 6
Statistical source data
Source Data Extended Data Fig. 6
Unprocessed gels
Source Data Extended Data Fig. 7
Statistical source data
Source Data Extended Data Fig. 7
Unprocessed gels
Source Data Extended Data Fig. 8
Statistical source data
Source Data Extended Data Fig. 8
Unprocessed gels
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, YY., Li, BY., Xiao, G. et al. Cdo1 promotes PPARγ-mediated adipose tissue lipolysis in male mice. Nat Metab 4, 1352–1368 (2022). https://doi.org/10.1038/s42255-022-00644-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00644-3
This article is cited by
-
Cdo1-Camkk2-AMPK axis confers the protective effects of exercise against NAFLD in mice
Nature Communications (2023)