Abstract
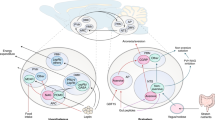
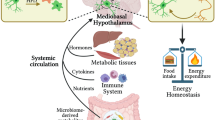
Communication between the periphery and the brain is key for maintaining energy homeostasis. To do so, peripheral signals from the circulation reach the brain via the circumventricular organs (CVOs), which are characterized by fenestrated vessels lacking the protective blood–brain barrier (BBB). Glial cells, by virtue of their plasticity and their ideal location at the interface of blood vessels and neurons, participate in the integration and transmission of peripheral information to neuronal networks in the brain for the neuroendocrine control of whole-body metabolism. Metabolic diseases, such as obesity and type 2 diabetes, can disrupt the brain-to-periphery communication mediated by glial cells, highlighting the relevance of these cell types in the pathophysiology of such complications. An improved understanding of how glial cells integrate and respond to metabolic and humoral signals has become a priority for the discovery of promising therapeutic strategies to treat metabolic disorders. This Review highlights the role of glial cells in the exchange of metabolic signals between the periphery and the brain that are relevant for the regulation of whole-body energy homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Garcia-Caceres, C. et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 22, 7–14 (2019).
Chowen, J. A., Frago, L. M. & Fernandez-Alfonso, M. S. Physiological and pathophysiological roles of hypothalamic astrocytes in metabolism. J. Neuroendocrinol. 31, e12671 (2019).
Clasadonte, J. & Prevot, V. The special relationship: glia–neuron interactions in the neuroendocrine hypothalamus. Nat. Rev. Endocrinol. 14, 25–44 (2018).
Al Massadi, O., Lopez, M., Tschop, M., Dieguez, C. & Nogueiras, R. Current understanding of the hypothalamic ghrelin pathways inducing appetite and adiposity. Trends Neurosci. 40, 167–180 (2017).
Friedman, J. M. Leptin and the endocrine control of energy balance. Nat. Metab. 1, 754–764 (2019).
Campbell, J. E. & Newgard, C. B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 22, 142–158 (2021).
Prevot, V. et al. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr. Rev. 39, 333–368 (2018).
Yeo, G. S. & Heisler, L. K. Unraveling the brain regulation of appetite: lessons from genetics. Nat. Neurosci. 15, 1343–1349 (2012).
Cone, R. D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 8, 571–578 (2005).
Dubern, B. et al. Mutational analysis of the pro-opiomelanocortin gene in French obese children led to the identification of a novel deleterious heterozygous mutation located in the α-melanocyte stimulating hormone domain. Pediatr. Res. 63, 211–216 (2008).
Chowen, J. A. et al. The role of astrocytes in the hypothalamic response and adaptation to metabolic signals. Prog. Neurobiol. 144, 68–87 (2016).
Kim, J. G. et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 17, 908–910 (2014).
Varela, L. et al. Hunger-promoting AgRP neurons trigger an astrocyte-mediated feed-forward autoactivation loop in mice. J. Clin. Invest. 131, e144239 (2021).
Garcia-Caceres, C. et al. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166, 867–880 (2016).
Gao, Y. et al. Disruption of lipid uptake in astroglia exacerbates diet-induced obesity. Diabetes 66, 2555–2563 (2017).
Varela, L. et al. Astrocytic lipid metabolism determines susceptibility to diet-induced obesity. Sci. Adv. 7, eabj2814 (2021).
Kreft, M., Bak, L. K., Waagepetersen, H. S. & Schousboe, A. Aspects of astrocyte energy metabolism, amino acid neurotransmitter homoeostasis and metabolic compartmentation. ASN Neuro 4, e00086 (2012).
Bonvento, G. & Bolanos, J. P. Astrocyte–neuron metabolic cooperation shapes brain activity. Cell Metab. 33, 1546–1564 (2021).
Obradovic, M. et al. Leptin and obesity: role and clinical implication. Front Endocrinol. 12, 585887 (2021).
Glaum, S. R. et al. Leptin, the obese gene product, rapidly modulates synaptic transmission in the hypothalamus. Mol. Pharmacol. 50, 230–235 (1996).
Garcia-Caceres, C. et al. Differential acute and chronic effects of leptin on hypothalamic astrocyte morphology and synaptic protein levels. Endocrinology 152, 1809–1818 (2011).
Fuente-Martin, E. et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J. Clin. Invest. 122, 3900–3913 (2012).
Banks, W. A. The blood–brain barrier as an endocrine tissue. Nat. Rev. Endocrinol. 15, 444–455 (2019).
Duquenne, M. et al. Leptin brain entry via a tanycytic LepR–EGFR shuttle controls lipid metabolism and pancreas function. Nat. Metab. 3, 1071–1090 (2021).
Balland, E. et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 19, 293–301 (2014).
Butiaeva, L. I. et al. Leptin receptor-expressing pericytes mediate access of hypothalamic feeding centers to circulating leptin. Cell Metab. 33, 1433–1448 (2021).
Djogo, T. et al. Adult NG2–glia are required for median eminence-mediated leptin sensing and body weight control. Cell Metab. 23, 797–810 (2016).
Muller, T. D. et al. Ghrelin. Mol. Metab. 4, 437–460 (2015).
Chen, N. et al. Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. eLife 5, e18716 (2016).
Clasadonte, J. et al. Prostaglandin E2 release from astrocytes triggers gonadotropin-releasing hormone (GnRH) neuron firing via EP2 receptor activation. Proc. Natl Acad. Sci. USA 108, 16104–16109 (2011).
Lebrun, B. et al. Glial endozepines and energy balance: old peptides with new tricks. Glia 69, 1079–1093 (2021).
Bouyakdan, K. et al. The gliotransmitter ACBP controls feeding and energy homeostasis via the melanocortin system. J. Clin. Invest. 129, 2417–2430 (2019).
Pellerin, L. & Magistretti, P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl Acad. Sci. USA 91, 10625–10629 (1994).
Bergersen, L. H. Lactate transport and signaling in the brain: potential therapeutic targets and roles in body–brain interaction. J. Cereb. Blood Flow. Metab. 35, 176–185 (2015).
Rafiki, A., Boulland, J. L., Halestrap, A. P., Ottersen, O. P. & Bergersen, L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience 122, 677–688 (2003).
Lhomme, T. et al. Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons. J. Clin. Invest. 131, e140521 (2021).
Ordenes, P. et al. Lactate activates hypothalamic POMC neurons by intercellular signaling. Sci. Rep. 11, 21644 (2021).
Tirou, L. et al. Sonic Hedgehog receptor Patched deficiency in astrocytes enhances glucose metabolism in mice. Mol. Metab. 47, 101172 (2021).
Nuzzaci, D. et al. Postprandial hyperglycemia stimulates neuroglial plasticity in hypothalamic POMC neurons after a balanced meal. Cell Rep. 30, 3067–3078 (2020).
Scharbarg, E. et al. Astrocyte-derived adenosine is central to the hypnogenic effect of glucose. Sci. Rep. 6, 19107 (2016).
Benani, A. et al. Food intake adaptation to dietary fat involves PSA-dependent rewiring of the arcuate melanocortin system in mice. J. Neurosci. 32, 11970–11979 (2012).
Allard, C. et al. Hypothalamic astroglial connexins are required for brain glucose sensing-induced insulin secretion. J. Cereb. Blood Flow. Metab. 34, 339–346 (2014).
Giaume, C., Leybaert, L., Naus, C. C. & Saez, J. C. Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front. Pharm. 4, 88 (2013).
Cheung, G., Chever, O. & Rouach, N. Connexons and pannexons: newcomers in neurophysiology. Front. Cell Neurosci. 8, 348 (2014).
Clasadonte, J., Scemes, E., Wang, Z., Boison, D. & Haydon, P. G. Connexin 43-mediated astroglial metabolic networks contribute to the regulation of the sleep–wake cycle. Neuron 95, 1365–1380 (2017).
Fioramonti, X. et al. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes 56, 1219–1227 (2007).
Song, Z., Levin, B. E., McArdle, J. J., Bakhos, N. & Routh, V. H. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 50, 2673–2681 (2001).
Claret, M. et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Invest. 117, 2325–2336 (2007).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012).
Douglass, J. D., Dorfman, M. D., Fasnacht, R., Shaffer, L. D. & Thaler, J. P. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol. Metab. 6, 366–373 (2017).
Cai, D. & Khor, S. Hypothalamic microinflammation. Handb. Clin. Neurol. 181, 311–322 (2021).
Zhang, X. et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Kleinridders, A. et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 10, 249–259 (2009).
Benzler, J. et al. Central inhibition of IKKβ/NF-κB signaling attenuates high-fat diet-induced obesity and glucose intolerance. Diabetes 64, 2015–2027 (2015).
Zhang, Y., Reichel, J. M., Han, C., Zuniga-Hertz, J. P. & Cai, D. Astrocytic process plasticity and IKKβ/NF-κB in central control of blood glucose, blood pressure, and body weight. Cell Metab. 25, 1091–1102 (2017).
Rahman, M. H. et al. Astrocytic pyruvate dehydrogenase kinase-2 is involved in hypothalamic inflammation in mouse models of diabetes. Nat. Commun. 11, 5906 (2020).
Campbell, J. N. et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 20, 484–496 (2017).
Prevot, V., Nogueiras, R. & Schwaninger, M. Tanycytes in the infundibular nucleus and median eminence and their role in the blood–brain barrier. Handb. Clin. Neurol. 180, 253–273 (2021).
Nampoothiri, S., Duquenne, M. & Prevot, V. in Glial-Neuronal Signaling in Neuroendocrine Systems, Vol. 11 (eds. Tasker, J. G., Bains, J. S. & Chowen, J. A.) 255–284 (Springer Cham, 2021).
Lee, D. A. et al. Dietary and sex-specific factors regulate hypothalamic neurogenesis in young adult mice. Front. Neurosci. 8, 157 (2014).
Sharif, A., Fitzsimons, C. P. & Lucassen, P. J. Neurogenesis in the adult hypothalamus: a distinct form of structural plasticity involved in metabolic and circadian regulation, with potential relevance for human pathophysiology. Handb. Clin. Neurol. 179, 125–140 (2021).
Haan, N. et al. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J. Neurosci. 33, 6170–6180 (2013).
Langlet, F. et al. Tanycytic VEGF-A boosts blood–hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 17, 607–617 (2013).
Mullier, A., Bouret, S. G., Prevot, V. & Dehouck, B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood–hypothalamus barrier regulation in the adult mouse brain. J. Comp. Neurol. 518, 943–962 (2010).
Schaeffer, M. et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc. Natl Acad. Sci. USA 110, 1512–1517 (2013).
Jiang, H. et al. MCH neurons regulate permeability of the median eminence barrier. Neuron 107, 306–319 (2020).
Caron, E., Sachot, C., Prevot, V. & Bouret, S. G. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J. Comp. Neurol. 518, 459–476 (2010).
Caro, J. F. et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348, 159–161 (1996).
Schwartz, M. W., Peskind, E., Raskind, M., Boyko, E. J. & Porte, D. Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat. Med. 2, 589–593 (1996).
Chmielewski, A. et al. Preclinical assessment of leptin transport into the cerebrospinal fluid in diet-induced obese minipigs. Obesity 27, 950–956 (2019).
Frederich, R. C. et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1, 1311–1314 (1995).
Hummel, K. P., Dickie, M. M. & Coleman, D. L. Diabetes, a new mutation in the mouse. Science 153, 1127–1128 (1966).
Wyse, B. M. & Dulin, W. E. The influence of age and dietary conditions on diabetes in the db mouse. Diabetologia 6, 268–273 (1970).
Lee, G. H. et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379, 632–635 (1996).
Guillebaud, F. et al. Glial endozepines reverse high-fat diet-induced obesity by enhancing hypothalamic response to peripheral leptin. Mol. Neurobiol. 57, 3307–3333 (2020).
Yoo, S., Cha, D., Kim, D. W., Hoang, T. V. & Blackshaw, S. Tanycyte-independent control of hypothalamic leptin signaling. Front. Neurosci. 13, 240 (2019).
Porniece Kumar, M. et al. Insulin signalling in tanycytes gates hypothalamic insulin uptake and regulation of AgRP neuron activity. Nat. Metab. 3, 1662–1679 (2021).
Collden, G. et al. Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Mol. Metab. 4, 15–24 (2015).
Uriarte, M. et al. Circulating ghrelin crosses the blood–cerebrospinal fluid barrier via growth hormone secretagogue receptor dependent and independent mechanisms. Mol. Cell. Endocrinol. 538, 111449 (2021).
Liang, Q. et al. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 63, 4064–4075 (2014).
Owen, B. M. et al. FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med. 19, 1153–1156 (2013).
Xu, C. et al. KLB, encoding β-Klotho, is mutated in patients with congenital hypogonadotropic hypogonadism. EMBO Mol. Med. 9, 1379–1397 (2017).
Pena-León, V. et al. Prolonged breastfeeding protects from obesity by hypothalamic action of hepatic FGF21. Nat. Metab. (in the press).
Kaminskas, B. et al. Characterisation of endogenous players in fibroblast growth factor-regulated functions of hypothalamic tanycytes and energy-balance nuclei. J. Neuroendocrinol. 31, e12750 (2019).
Bottcher, M. et al. NF-κB signaling in tanycytes mediates inflammation-induced anorexia. Mol. Metab. 39, 101022 (2020).
Frayling, C., Britton, R. & Dale, N. ATP-mediated glucosensing by hypothalamic tanycytes. J. Physiol. 589, 2275–2286 (2011).
Lazutkaite, G., Solda, A., Lossow, K., Meyerhof, W. & Dale, N. Amino acid sensing in hypothalamic tanycytes via umami taste receptors. Mol. Metab. 6, 1480–1492 (2017).
Hofmann, K. et al. Tanycytes and a differential fatty acid metabolism in the hypothalamus. Glia 65, 231–249 (2017).
Geller, S. et al. Tanycytes regulate lipid homeostasis by sensing free fatty acids and signaling to key hypothalamic neuronal populations via FGF21 secretion. Cell Metab. 30, 833–844 e837 (2019).
Benford, H. et al. A sweet taste receptor-dependent mechanism of glucosensing in hypothalamic tanycytes. Glia 65, 773–789 (2017).
Sanders, N. M., Dunn-Meynell, A. A. & Levin, B. E. Third ventricular alloxan reversibly impairs glucose counterregulatory responses. Diabetes 53, 1230–1236 (2004).
Haddad-Tovolli, R. & Claret, M. Cooperative tanycytes fuel the neuronal tank. J. Clin. Invest. 131, e153279 (2021).
Bolborea, M., Pollatzek, E., Benford, H., Sotelo-Hitschfeld, T. & Dale, N. Hypothalamic tanycytes generate acute hyperphagia through activation of the arcuate neuronal network. Proc. Natl Acad. Sci. USA 117, 14473–14481 (2020).
Cortes-Campos, C. et al. MCT expression and lactate influx/efflux in tanycytes involved in glia–neuron metabolic interaction. PLoS ONE 6, e16411 (2011).
Conductier, G. et al. Melanin-concentrating hormone regulates beat frequency of ependymal cilia and ventricular volume. Nat. Neurosci. 16, 845–847 (2013).
Rodriguez-Cortes, B. et al. Suprachiasmatic nucleus-mediated glucose entry into the arcuate nucleus determines the daily rhythm in blood glycemia. Curr. Biol. 32, 796–805 (2022).
Imbernon, M., Dehouck, B. & Prevot, V. Glycemic control: tanycytes march to the beat of the suprachiasmatic drummer. Curr. Biol. 32, R173–R176 (2022).
Marcelin, G. et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol. Metab. 3, 19–28 (2014).
Brown, J. M. et al. Role of hypothalamic MAPK/ERK signaling and central action of FGF1 in diabetes remission. iScience 24, 102944 (2021).
Bentsen, M. A. et al. Transcriptomic analysis links diverse hypothalamic cell types to fibroblast growth factor 1-induced sustained diabetes remission. Nat. Commun. 11, 4458 (2020).
Scarlett, J. M. et al. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat. Med. 22, 800–806 (2016).
Brown, J. M. et al. The hypothalamic arcuate nucleus-median eminence is a target for sustained diabetes remission induced by fibroblast growth factor 1. Diabetes 68, 1054–1061 (2019).
Samms, R. J. et al. Antibody-mediated inhibition of the FGFR1c isoform induces a catabolic lean state in Siberian hamsters. Curr. Biol. 25, 2997–3003 (2015).
Kim, S. et al. Tanycytic TSPO inhibition induces lipophagy to regulate lipid metabolism and improve energy balance. Autophagy 16, 1200–1220 (2020).
Drucker, D. J., Habener, J. F. & Holst, J. J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Invest. 127, 4217–4227 (2017).
Imbernon, M. et al. Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metab. 34, 1054–1063.e7 (2022).
Gabery, S. et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 5, e133429 (2020).
Rohrbach, A. et al. Ablation of glucokinase-expressing tanycytes impacts energy balance and increases adiposity in mice. Mol. Metab. 53, 101311 (2021).
Yoo, S. et al. Tanycyte ablation in the arcuate nucleus and median eminence increases obesity susceptibility by increasing body fat content in male mice. Glia 68, 1987–2000 (2020).
Fekete, C. & Lechan, R. M. Central regulation of hypothalamic–pituitary–thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 35, 159–194 (2014).
Muller-Fielitz, H. et al. Tanycytes control the hormonal output of the hypothalamic–pituitary–thyroid axis. Nat. Commun. 8, 484 (2017).
Farkas, E. et al. A glial–neuronal circuit in the median eminence regulates thyrotropin-releasing hormone-release via the endocannabinoid system. iScience 23, 100921 (2020).
Goodman, T. & Hajihosseini, M. K. Hypothalamic tanycytes-masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front. Neurosci. 9, 387 (2015).
Yoo, S. & Blackshaw, S. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog. Neurobiol. 170, 53–66 (2018).
Weiss, S. et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J. Neurosci. 16, 7599–7609 (1996).
Li, J., Tang, Y. & Cai, D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol. 14, 999–1012 (2012).
Robins, S. C. et al. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun. 4, 2049 (2013).
Goodman, T. et al. Fibroblast growth factor 10 is a negative regulator of postnatal neurogenesis in the mouse hypothalamus. Development 147, dev180950 (2020).
Recabal, A. et al. The FGF2-induced tanycyte proliferation involves a connexin 43 hemichannel/purinergic-dependent pathway. J. Neurochem. 156, 182–199 (2021).
Orellana, J. A. et al. Glucose increases intracellular free Ca2+ in tanycytes via ATP released through connexin 43 hemichannels. Glia 60, 53–68 (2012).
Lee, D. A. et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 15, 700–702 (2012).
Yoo, S. et al. Control of neurogenic competence in mammalian hypothalamic tanycytes. Sci. Adv. 7, eabg3777 (2021).
Mu, W. et al. Hypothalamic Rax+ tanycytes contribute to tissue repair and tumorigenesis upon oncogene activation in mice. Nat. Commun. 12, 2288 (2021).
Surbhi, Wittmann, G., Low, M. J. & Lechan, R. M. Adult-born proopiomelanocortin neurons derived from Rax-expressing precursors mitigate the metabolic effects of congenital hypothalamic proopiomelanocortin deficiency. Mol. Metab. 53, 101312 (2021).
Son, J. E. et al. Irx3 and Irx5 in Ins2–Cre+ cells regulate hypothalamic postnatal neurogenesis and leptin response. Nat. Metab. 3, 701–713 (2021).
Dou, Z., Son, J. E. & Hui, C. C. Irx3 and Irx5 — novel regulatory factors of postnatal hypothalamic neurogenesis. Front Neurosci. 15, 763856 (2021).
Mirzadeh, Z. et al. Bi- and uniciliated ependymal cells define continuous floor-plate-derived tanycytic territories. Nat. Commun. 8, 13759 (2017).
Faubel, R., Westendorf, C., Bodenschatz, E. & Eichele, G. Cilia-based flow network in the brain ventricles. Science 353, 176–178 (2016).
Genzen, J. R., Yang, D., Ravid, K. & Bordey, A. Activation of adenosine A2B receptors enhances ciliary beat frequency in mouse lateral ventricle ependymal cells. Cerebrospinal Fluid Res. 6, 15 (2009).
Genzen, J. R., Platel, J. C., Rubio, M. E. & Bordey, A. Ependymal cells along the lateral ventricle express functional P2X(7) receptors. Purinergic Signal 5, 299–307 (2009).
Conductier, G. et al. Control of ventricular ciliary beating by the melanin concentrating hormone-expressing neurons of the lateral hypothalamus: a functional imaging survey. Front. Endocrinol. 4, 182 (2013).
Noble, E. E. et al. Control of feeding behavior by cerebral ventricular volume transmission of melanin-concentrating hormone. Cell Metab. 28, 55–68 e57 (2018).
Al-Massadi, O. et al. Multifaceted actions of melanin-concentrating hormone on mammalian energy homeostasis. Nat. Rev. Endocrinol. 17, 745–755 (2021).
Zilkha-Falb, R., Kaushansky, N. & Ben-Nun, A. The median eminence, a new oligodendrogenic niche in the adult mouse brain. Stem Cell Rep. 14, 1076–1092 (2020).
Marsters, C. M. et al. Oligodendrocyte development in the embryonic tuberal hypothalamus and the influence of Ascl1. Neural Dev. 11, 20 (2016).
Kohnke, S. et al. Nutritional regulation of oligodendrocyte differentiation regulates perineuronal net remodeling in the median eminence. Cell Rep. 36, 109362 (2021).
Ren, Z. et al. Conditional knockout of leptin receptor in neural stem cells leads to obesity in mice and affects neuronal differentiation in the hypothalamus early after birth. Mol. Brain 13, 109 (2020).
Alonge, K. M. et al. Hypothalamic perineuronal net assembly is required for sustained diabetes remission induced by fibroblast growth factor 1 in rats. Nat. Metab. 2, 1025–1033 (2020).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Saab, A. S. et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132 (2016).
Ou, Z. et al. A GPR17–cAMP–lactate signaling axis in oligodendrocytes regulates whole-body metabolism. Cell Rep. 26, 2984–2997 (2019).
Ren, H. et al. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell 149, 1314–1326 (2012).
Ren, H., Cook, J. R., Kon, N. & Accili, D. Gpr17 in AgRP neurons regulates feeding and sensitivity to insulin and leptin. Diabetes 64, 3670–3679 (2015).
Le Thuc, O. et al. Hypothalamic inflammation and energy balance disruptions: spotlight on chemokines. Front Endocrinol. 8, 197 (2017).
Wisse, B. E. et al. Evidence that lipopolysaccharide-induced anorexia depends upon central, rather than peripheral, inflammatory signals. Endocrinology 148, 5230–5237 (2007).
Jang, P. G. et al. NF-κB activation in hypothalamic pro-opiomelanocortin neurons is essential in illness- and leptin-induced anorexia. J. Biol. Chem. 285, 9706–9715 (2010).
Jin, S. et al. Hypothalamic TLR2 triggers sickness behavior via a microglia–neuronal axis. Sci. Rep. 6, 29424 (2016).
Le Thuc, O. et al. Central CCL2 signaling onto MCH neurons mediates metabolic and behavioral adaptation to inflammation. EMBO Rep. 17, 1738–1752 (2016).
Pan, W. et al. Cytokine signaling modulates blood–brain barrier function. Curr. Pharm. Des. 17, 3729–3740 (2011).
Banks, W. A. Anorectic effects of circulating cytokines: role of the vascular blood–brain barrier. Nutrition 17, 434–437 (2001).
Valdearcos, M. et al. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 9, 2124–2138 (2014).
Kim, F. et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler. Thromb. Vasc. Biol. 28, 1982–1988 (2008).
Zhang, G. et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497, 211–216 (2013).
Heiss, C. N. et al. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 35, 109163 (2021).
Kim, J. D., Yoon, N. A., Jin, S. & Diano, S. Microglial UCP2 mediates inflammation and obesity induced by high-fat feeding. Cell Metab. 30, 952–962 e955 (2019).
Dorfman, M. D. et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun. 8, 14556 (2017).
Cansell, C. et al. Dietary fat exacerbates postprandial hypothalamic inflammation involving glial fibrillary acidic protein-positive cells and microglia in male mice. Glia 69, 42–60 (2021).
Chowen, J. A., Argente-Arizon, P., Freire-Regatillo, A. & Argente, J. Sex differences in the neuroendocrine control of metabolism and the implication of astrocytes. Front. Neuroendocrinol. 48, 3–12 (2018).
Binder, A. K. et al. Steroid Receptors in the Uterus and Ovary (Academic Press, 2015).
Giacobini, P. et al. Brain endothelial cells control fertility through ovarian-steroid-dependent release of semaphorin 3A. PLoS Biol. 12, e1001808 (2014).
Prevot, V., Cornea, A., Mungenast, A., Smiley, G. & Ojeda, S. R. Activation of erbB-1 signaling in tanycytes of the median eminence stimulates transforming growth factor β1 release via prostaglandin E2 production and induces cell plasticity. J. Neurosci. 23, 10622–10632 (2003).
Asarian, L. & Geary, N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1251–1263 (2006).
Prevot, V. et al. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J. Neurosci. 23, 230–239 (2003).
Pellegrino, G. et al. GnRH neurons recruit astrocytes in infancy to facilitate network integration and sexual maturation. Nat. Neurosci. 24, 1660–1672 (2021).
Zeng, F., Wang, Y., Kloepfer, L. A., Wang, S. & Harris, R. C. ErbB4 deletion predisposes to development of metabolic syndrome in mice. Am. J. Physiol. Endocrinol. Metab. 315, E583–E593 (2018).
Day, F. R. et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat. Commun. 6, 8464 (2015).
Prevot, V. & Sharif, A. The polygamous GnRH neuron: astrocytic and tanycytic communication with a neuroendocrineneuronal population. J. Neuroendocrinol. 34, e13104 (2020).
Swamydas, M., Bessert, D. & Skoff, R. Sexual dimorphism of oligodendrocytes is mediated by differential regulation of signaling pathways. J. Neurosci. Res. 87, 3306–3319 (2009).
Cerghet, M. et al. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J. Neurosci. 26, 1439–1447 (2006).
Yasuda, K. et al. Sex-specific differences in transcriptomic profiles and cellular characteristics of oligodendrocyte precursor cells. Stem Cell Res. 46, 101866 (2020).
Marraudino, M. et al. G-protein-coupled estrogen receptor immunoreactivity in the rat hypothalamus is widely distributed in neurons, astrocytes, and oligodendrocytes, fluctuates during the estrous cycle, and is sexually dimorphic. Neuroendocrinology 111, 660–677 (2021).
Villa, A. et al. Sex-specific features of microglia from adult mice. Cell Rep. 23, 3501–3511 (2018).
Guneykaya, D. et al. Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 24, 2773–2783 (2018).
Tramunt, B. et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 63, 453–461 (2020).
Ludwig, M. Q. et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat. Metab. 3, 530–545 (2021).
Ludwig, M. Q., Todorov, P. V., Egerod, K. L., Olson, D. P. & Pers, T. H. Single-cell mapping of GLP-1 and GIP receptor expression in the dorsal vagal complex. Diabetes 70, 1945–1955 (2021).
Filippi, B. M. et al. Insulin signals through the dorsal vagal complex to regulate energy balance. Diabetes 63, 892–899 (2014).
Hayes, M. R. et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 11, 77–83 (2010).
Williams, E. K. et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Muller, T. D. et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 30, 72–130 (2019).
Grill, H. J. & Hayes, M. R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 16, 296–309 (2012).
Hayes, M. R., Mietlicki-Baase, E. G., Kanoski, S. E. & De Jonghe, B. C. Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu. Rev. Nutr. 34, 237–260 (2014).
Reiner, D. J. et al. Astrocytes regulate GLP-1 receptor-mediated effects on energy balance. J. Neurosci. 36, 3531–3540 (2016).
Yulyaningsih, E. et al. Acute lesioning and rapid repair of hypothalamic neurons outside the blood–brain barrier. Cell Rep. 19, 2257–2271 (2017).
Luo, L. et al. Optimizing nervous system-specific gene targeting with cre driver lines: prevalence of germline recombination and influencing factors. Neuron 106, 37–65 (2020).
Pak, T., Yoo, S., Miranda-Angulo, A. L., Wang, H. & Blackshaw, S. Rax–CreERT2 knock-in mice: a tool for selective and conditional gene deletion in progenitor cells and radial glia of the retina and hypothalamus. PLoS ONE 9, e90381 (2014).
Zhou, Y. et al. Author Correction: Temporal dynamic reorganization of 3D chromatin architecture in hormone-induced breast cancer and endocrine resistance. Nat. Commun. 11, 1967 (2020).
Magnani, L. et al. Genome-wide reprogramming of the chromatin landscape underlies endocrine therapy resistance in breast cancer. Proc. Natl Acad. Sci. USA 110, E1490–E1499 (2013).
Liu, Z. et al. Short-term tamoxifen treatment has long-term effects on metabolism in high-fat diet-fed mice with involvement of Nmnat2 in POMC neurons. FEBS Lett. 592, 3305–3316 (2018).
O’Carroll, S. J., Cook, W. H. & Young, D. AAV targeting of glial cell types in the central and peripheral nervous system and relevance to human gene therapy. Front Mol. Neurosci. 13, 618020 (2020).
Howard, D. B., Powers, K., Wang, Y. & Harvey, B. K. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology 372, 24–34 (2008).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Zhu, H. et al. Cre-dependent DREADD (designer receptors exclusively activated by designer drugs) mice. Genesis 54, 439–446 (2016).
Acknowledgements
This work was supported by the European Research Council (ERC) Synergy Grant WATCH (Well Aging and the Tanycytic Control of Health), No 810331 to R. N., V. P. and M. S.
Author information
Authors and Affiliations
Contributions
S. N. and V. P. designed the structure of the Review. S. N. wrote the first draft. R. N., M. S. and V. P. discussed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nampoothiri, S., Nogueiras, R., Schwaninger, M. et al. Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis. Nat Metab 4, 813–825 (2022). https://doi.org/10.1038/s42255-022-00610-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00610-z
This article is cited by
-
Glucose dysregulation in antipsychotic-naive first-episode psychosis: in silico exploration of gene expression signatures
Translational Psychiatry (2024)
-
Metabolic control of puberty: 60 years in the footsteps of Kennedy and Mitra’s seminal work
Nature Reviews Endocrinology (2024)
-
Oligodendrocyte progenitor cells in Alzheimer’s disease: from physiology to pathology
Translational Neurodegeneration (2023)