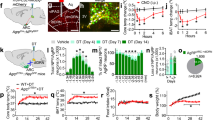
Abstract
Phospholipid levels are influenced by peripheral metabolism. Within the central nervous system, synaptic phospholipids regulate glutamatergic transmission and cortical excitability. Whether changes in peripheral metabolism affect brain lipid levels and cortical excitability remains unknown. Here, we show that levels of lysophosphatidic acid (LPA) species in the blood and cerebrospinal fluid are elevated after overnight fasting and lead to higher cortical excitability. LPA-related cortical excitability increases fasting-induced hyperphagia, and is decreased following inhibition of LPA synthesis. Mice expressing a human mutation (Prg-1R346T) leading to higher synaptic lipid-mediated cortical excitability display increased fasting-induced hyperphagia. Accordingly, human subjects with this mutation have higher body mass index and prevalence of type 2 diabetes. We further show that the effects of LPA following fasting are under the control of hypothalamic agouti-related peptide (AgRP) neurons. Depletion of AgRP-expressing cells in adult mice decreases fasting-induced elevation of circulating LPAs, as well as cortical excitability, while blunting hyperphagia. These findings reveal a direct influence of circulating LPAs under the control of hypothalamic AgRP neurons on cortical excitability, unmasking an alternative non-neuronal route by which the hypothalamus can exert a robust impact on the cortex and thereby affect food intake.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of our study can be found in the Source Data provided with this article. Expression data of Enpp2/Atx and Prg-1/Lppr4 were analysed using the Allen Mouse Brain Atlas.
References
Trimbuch, T. et al. Synaptic PRG-1 modulates excitatory transmission via lipid phosphate-mediated signaling. Cell 138, 1222–1235 (2009).
Unichenko, P. et al. Plasticity-related gene 1 affects mouse barrel cortex function via strengthening of glutamatergic thalamocortical transmission. Cereb. Cortex 26, 3260–3272 (2016).
Vogt, J. et al. Molecular cause and functional impact of altered synaptic lipid signaling due to a prg-1 gene SNP. EMBO Mol. Med. 8, 25–38 (2016).
Thalman, C. et al. Synaptic phospholipids as a new target for cortical hyperexcitability and E/I balance in psychiatric disorders. Mol. Psychiatry 23, 1699–1710 (2018).
Moolenaar, W. H., van Meeteren, L. A. & Giepmans, B. N. The ins and outs of lysophosphatidic acid signaling. BioEssays 26, 870–881 (2004).
Yung, Y. C., Stoddard, N. C., Mirendil, H. & Chun, J. Lysophosphatidic acid signaling in the nervous system. Neuron 85, 669–682 (2015).
Yung, Y. C., Stoddard, N. C. & Chun, J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J. Lipid Res. 55, 1192–1214 (2014).
Tang, X., Benesch, M. G. & Brindley, D. N. Lipid phosphate phosphatases and their roles in mammalian physiology and pathology. J. Lipid Res. 56, 2048–2060 (2015).
Moolenaar, W. H. Lysophospholipids in the limelight: autotaxin takes center stage. J. Cell Biol. 158, 197–199 (2002).
Stracke, M. L. et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem. 267, 2524–2529 (1992).
Hausmann, J. et al. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat. Struct. Mol. Biol. 18, 198–204 (2011).
Sekas, G., Patton, G. M., Lincoln, E. C. & Robins, S. J. Origin of plasma lysophosphatidylcholine: evidence for direct hepatic secretion in the rat. J. Lab. Clin. Med. 105, 190–194 (1985).
Brindley. Hepatic secretion of lysophosphatidylcholine: a novel transport system for polyunsaturated fatty acids and choline. J. Nutr. Biochem. 4, 442–449 (1993).
Nguyen, L. N. et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509, 503–506 (2014).
Nakamura, K. et al. Autotaxin enzyme immunoassay in human cerebrospinal fluid samples. Clin. Chim. Acta 405, 160–162 (2009).
Sato, K. et al. Identification of autotaxin as a neurite retraction-inducing factor of PC12 cells in cerebrospinal fluid and its possible sources. J. Neurochem. 92, 904–914 (2005).
Geisler, C. E., Hepler, C., Higgins, M. R. & Renquist, B. J. Hepatic adaptations to maintain metabolic homeostasis in response to fasting and refeeding in mice. Nutr. Metab. (Lond.) 13, 62 (2016).
Joly-Amado, A. et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 31, 4276–4288 (2012).
Miletta, M. C. et al. AgRP neurons control compulsive exercise and survival in an activity-based anorexia model. Nat. Metab. 2, 1204–1211 (2020).
Dietrich, M. O. et al. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat. Neurosci. 15, 1108–1110 (2012).
Dietrich, M. O., Zimmer, M. R., Bober, J. & Horvath, T. L. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 160, 1222–1232 (2015).
Schmitz, K. et al. Dysregulation of lysophosphatidic acids in multiple sclerosis and autoimmune encephalomyelitis. Acta Neuropathol. Commun. 5, 42 (2017).
Kano, K. et al. Molecular mechanism of lysophosphatidic acid-induced hypertensive response. Sci. Rep. 9, 2662 (2019).
Leger, M. et al. Object recognition test in mice. Nat. Protoc. 8, 2531–2537 (2013).
Fernandez, G. et al. Evidence supporting a role for constitutive ghrelin receptor signaling in fasting-induced hyperphagia in male mice. Endocrinology 159, 1021–1034 (2018).
He, Y. et al. A small potassium current in AgRP/NPY neurons regulates feeding behavior and energy metabolism. Cell. Rep. 17, 1807–1818 (2016).
Krizo, J. A. et al. Regulation of locomotor activity in fed, fasted, and food-restricted mice lacking tissue-type plasminogen activator. BMC. Physiol. 18, 2 (2018).
Dunlop, K. A., Woodside, B. & Downar, J. Targeting neural endophenotypes of eating disorders with non-invasive brain stimulation. Front. Neurosci. 10, 30 (2016).
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Kaluarachchi, M. et al. A comparison of human serum and plasma metabolites using untargeted (1)H NMR spectroscopy and UPLC-MS. Metabolomics 14, 32 (2018).
Tabbai, S. et al. Effects of the LPA1 receptor deficiency and stress on the hippocampal LPA species in mice. Front. Mol. Neurosci. 12, 146 (2019).
Oliveira, T. G. et al. The impact of chronic stress on the rat brain lipidome. Mol. Psychiatry 21, 80–88 (2016).
Tataranni, P. A. et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc. Natl Acad. Sci. USA 96, 4569–4574 (1999).
Cajal, S. R. Y. Textura del Sistema Nervioso del Hombre y de los Vertebrados (Nicolás Moya, 1899).
Waterson, M. J. & Horvath, T. L. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell. Metab. 22, 962–970 (2015).
Focker, M. et al. Evaluation of metabolic profiles of patients with anorexia nervosa at inpatient admission, short- and long-term weight regain-descriptive and pattern analysis. Metabolites https://doi.org/10.3390/metabo11010007 (2020).
Contos, J. J. et al. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol. Cell. Biol. 22, 6921–6929 (2002).
Fotopoulou, S. et al. ATX expression and LPA signalling are vital for the development of the nervous system. Dev. Biol. 339, 451–464 (2010).
Gorski, J. A. et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 22, 6309–6314 (2002).
Gierse, J. et al. A novel autotaxin inhibitor reduces lysophosphatidic acid levels in plasma and the site of inflammation. J. Pharmacol. Exp. Ther. 334, 310–317 (2010).
Brunkhorst-Kanaan, N. et al. Targeted lipidomics reveal derangement of ceramides in major depression and bipolar disorder. Metabolism 95, 65–76 (2019).
Johnson, V. E. Revised standards for statistical evidence. Proc. Natl Acad. Sci. USA 110, 19313–19317 (2013).
Acknowledgments
We thank C. Ernest for proofreading the manuscript. This work was supported by Deutsche Forschungsgemeinschaft (nos. FB 1039, 1080, 1193 and 1451) to J.V., R.N., I.T., F.Z. and S.G.; by Germany´s Excellence Strategy (no. EXC 2030, 390661388) to J.V.; by the European Research Council (nos. ERC-AG ‘LiPsyD’ and ERC-PoC ‘PsychAid’) to R.N.; by the Boehringer-Ingelheim Foundation to J.V. and S.G.; by Stiftung Rheinland-Pfalz to J.V., R.N. and F.Z.; by InfectControl (nos. 03ZZ0826 and 03ZZ0835 to Y.L. and F.K., respectively); by the German Research Foundation (FOR2107, nos. DA1151/5-1 and DA1151/5-2 to U.D.; SFB-TRR58, nos. C09 and Z02 to U.D.); and by the Interdisciplinary Center for Clinical Research of the Medical Faculty of Münster (no. Dan3/012/17 to U.D.). SHIP is part of the Community Medicine Research net of the University of Greifswald, which receives grants from the Federal Ministry of Education and Research (nos. 01ZZ9603, 01ZZ0103 and 01ZZ0403), the Ministry of Cultural Affairs and the Social Ministry of the Federal State of Mecklenburg-West Pomerania. Genome-wide SNP typing in SHIP has been supported by a joint grant from Siemens Healthineers, Erlangen and the Federal Ministry of Education and Research (no. 03ZIK012); by the European Union and European Social Fund grant (no. EFOP-3.6.2-16-2017-0008); by an NKFIH grant (no. KKP126998) to B.R.; and by NIH grant nos. AG052005, AG067329 and DK126447 and a Klarman Family Foundation Grant to T.L.H.
Author information
Authors and Affiliations
Contributions
J.V., R.N. and T.L.H. designed and supervised experiments and wrote the paper. Electrophysiological and behavioural experiments were performed by H.E., B.S., Z.-W.L., M.S.-P., B.R., J.B., W.F., N.S., G.H. and K.R. I.T., Y.S., I.F.S., R.G., F.K. and Y.L. performed mass spectrometry analyses and analysed data. M.M. was involved in data analysis and statistical analysis. R.N. and F.Z. founded the Gutenberg Brain Study, which provided human data. U.D. provided human data from both the Münster Neuroimaging Cohort and the FOR2107 consortium. H.J.G. provided human data from the Study of Health in Pomerania (Ship-O and Ship-Trend). I.T., N.O., S.G., F.Z. and U.D. provided important critical input to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.J.G. has received travel grants and speaker’s honoraria from Fresenius Medical Care, Neuraxpharm, Servier and Janssen Cilag, as well as research funding from Fresenius Medical Care. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Vytas Bankaitis, Scott E. Kanoski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 ATX and PRG-1 expression in cortical neurons.
a. Atx expression as shown by in-situ hybridization is present in the cortex and in the choroid ventricular plexus. Interestingly, strong expression in the cortex can be observed in the upper cortical layers (most prominent in layer II), which are important for cortical information processing. Image is adapted from the Allen Institute for Brain Science (2004). Allen Mouse Brain Atlas [532631] (mouse.brain-map.org). b-f. PRG-1 expression, as demonstrated by the ß-Gal reporter (for genetic details see Trimbuch et al., 2009), is predominantly found in the layers 2/3, 4 and 6 of the neocortex (visual cortex [A], auditory cortex [B], primary motor cortex [M1, C], dorsomedial prefrontal cortex [dmPFC,C] and primary sensory cortex [S1BF, F]). Strong expression was also found in the hippocampal formation (shown in E), as previously demonstrated. g-i. Staining for inhibitory neurons (GAD67, parvalbumin [PV] and calretinin [CR]) in the primary somatosensory cortex revealed no expression of PRG-1 in interneurons. Note the strong perisomatic inhibitory synapses of the excitatory, PRG-1-expressing neurons (arrowheads in the inserts in G and H). j. PRG-1 expression was detected in pyramidal (excitatory) neurons of the layer II, which express calbindin. Note the clear apical dendrites of these pyramidal neurons (arrowheads). PRG-1 expression as displayed in Fig. 1b-j was validated in at least 3 independent experiments. Scale bars: b-e 250 µm, f-j 100 µm, insets 15 µm.
Extended Data Fig. 2 LPA 18:1 is a synaptic active LPA.
a. Original traces of patched neurons before and after LPA 18:1 application suggest higher number of miniature inward currents (mEPSCs) when compared to values before LPA 18:1 application b. Quantitative analysis of mEPSC frequencies following LPA 18:1 application were significantly increased when compared to baseline values before LPA 18:1 application (n = 10; P = 0.0327, repeated measures one-way ANOVA). Data was calculated in % of the mean baseline over 3 min before drug application. c. For better evaluation, mean of the baseline of individual neurons over 3 min before LPA 18:1 stimulation was compared to that during the first 3 min after LPA 18:1 application finding significant differences (n = 10, P = 0.027, two-tailed Wilcoxon matched-pairs signed rank test). d. Original traces before and following of LPA 18:0 application show comparable mEPSCs. e,f. Quantitative analysis revealed no significant effect of LPA 18:0 application on mEPSC frequency (n = 9; repeated measures one-way ANOVA). Before and after LPA 18:0 application mean values of individual neurons were calculated as described above and shown in f (n = 8, two-tailed Wilcoxon matched-pairs signed rank test). Points and bars in b and e represent mean and s.e.m.
Extended Data Fig. 3 Fasting increases cortex-related exploratory behavior.
a. Experimental design. b. Fasting increased exploration (P < 0.0001), which was reduced to control values by ATX inhibition (P < 0.0001; n = 13 control mice [C], n = 14 fasted mice [F], and n = 12 PF8380-treated fasted mice [F + PF8380]; one-way ANOVA with Bonferroni correction). c. Cortical ATX-deletion (AtxΔcortex) did not affect exploration (n = 20 WT and 16 AtxΔcortex mice, two-tailed Mann-Whitney test) while it significantly reduced exploratory behavior after fasting (n = 21 fasted WT [F WT] and 19 fasted AtxΔcortex [F AtxΔcortex] mice; P = 0.0155, two-tailed t-test). d. Cortical ATX-deletion reduced fasting-induced exploration to non-fasting conditions (n = 16 non-fasted mice AtxΔcortex [C AtxΔcortex] mice and 16 fasted AtxΔcortex [F AtxΔcortex] mice, two-tailed t-test). e. ATX inhibition did not alter exploratory behavior of fasted AtxΔcortex mice (n = 6 fasted AtxΔcortex mice [F AtxΔcortex] and 8 fasted AtxΔcortex + PF8380 mice [F AtxΔcortex + PF8380], two-tailed t-test). f. Exploratory behavior of Lpa2-/- mice under baseline conditions and after fasting (n = 16 WT and n = 27 Lpa2-/- mice, two-tailed Mann-Whitney test; n = 11 fasted WT and 16 fasted Lpa2-/- [F Lpa2-/-] mice, P = 0.032, two-tailed t-test). g. ATX inhibition by PF8380 did not alter fasting-induced exploratory behavior in Lpa2-/- mice when compared to non-treated, fasted Lpa2-/- mice (n = 9 fasted Lpa2-/- mice [F Lpa2-/-] and 10 fasted Lpa2-/- +PF8380 [F Lpa2-/- +PF8380] mice, two-tailed Mann-Whitney test). h. Prg-1R346T/+ mice did show normal exploratory behavior under non-fasting conditions (n = 8 WT and n = 11 Prg-1R346T/+ mice, one-tailed t-test) while fasting significantly increased exploration (n = 20 fasted WT [F WT] and 23 fasted Prg-1R346T/+ mice [F Prg-1R346T/+]; P = 0.034, one-tailed t-test). Dots represent single values, bars represent mean and s.e.m., *p < 0.05, ****p < 0.0001. Illustration was created with BioRender.
Extended Data Fig. 4 Overnight fasting does not alter basic locomotion.
a. Experimental design. b. PF8380 i.p. application (30 mg/kg body weight) significantly reduced LPA subtype levels in the CSF. Note that some values in the CSF after ATX inhibition by PF8380 were below the lower level of quantification and set to 0 (n = 4 WT and 6 WT + PF8380 for LPA 16:0, n = 6 WT and WT + PF8380 for LPA 18:1, n = 5 WT and 6 WT + PF8380 for LPA 18:2 and LPA 20:4, group differences for LPA 16:0 97.6%, for LPA 18:1 99.2%, for LPA 18:2 84.5%, for LPA 20:4 69.9%,Bayesian analysis. Box plots and whiskers show data from min to max, line shows median; group differences * >80% or **>90%. c. Basic spontaneous motor function was not altered after fasting or after PF8380 treatment (n = 13 non-fasted WT [C], 15 fasted WT [F] and n = 15 fasted WT + PF8380 mice [F + PF8380], one-way ANOVA). d. Basic spontaneous motor function was not altered in AtxΔcortex mice under control conditions or after fasting [F] (n = 13 WT and fasted WT [F WT] mice and n = 12 AtxΔcortex mice [AtxΔcortex and F AtxΔcortex], two-tailed t-test). e. Basic spontaneous motor function was not altered in Lpa2-/- mice under control conditions or after fasting (n = 16 WT mice [WT and F WT] and n = 8 Lpa2-/- mice [Lpa2-/–and F Lpa2-/-], t-test). f. Basic spontaneous locomotion was not altered in Prg-1R346T/+ animals under control conditions or after fasting (n = 8 WT mice [WT and F WT] and n = 11 Prg-1R346T/+ mice [Prg-1R346T/+ and F Prg-1R346T/+], two-tailed Mann-Whitney test). Experimental design figures were created with BioRender.com. Dots show single values, bars show mean and s.e.m. Illustration was created with BioRender.
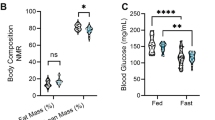
Extended Data Fig. 5 Altered synaptic lipid signaling is associated with increased body weight in mice and humans.
a. Fasting-induced hyperphagia was comparable in Prg-1+/- and Prg-1R346T/+ animals (n = 14 F Prg-1+/- and n = 13 F Prg-1R346T/+, two-sided t-test) b. Food consumption per hour of Prg-1R346T/+ and WT animals averaged over 24 hours (n = 20 WT and n = 23 Prg-1R346T/+, group differences 86,7%, Bayesian analysis). c. BMI was significantly increased in human PRG-1R345T/+ carriers (n = 58 PRG-1R346T/+ and n = 60 control subjects matched for sex, age, height and education, P = 0.0041, two-tailed Mann-Whitney test). d. Diabetes type 2 (DMT2) prevalence was significantly increased in male PRG-1R346T/+ carriers, which displayed a significantly higher odds ratio (OR) for DMT2 of 2.45 (p = 0.037, logistic regression adjusted for age, sex and cohort; n = 3947 control subjects with 443 individuals with diabetes; n = 32 PRG-1R345T/+ carriers with 7 individuals with diabetes; P = 0.0291, chi square test, p*<0.05). Graph shows number of diabetic individuals as a percentage of the corresponding group. i,j. PF8380 was quickly metabolized by human liver microsomes to a metabolite with 288 g∙mol-1 (n = 3; CLint > 100 µl ∙ (min ∙ mg)-1. Diclofenac was used as a positive control. Dots show single values, bars represent mean and SEM.*p < 0.05, ***p < 0.001, ****p < 0.0001, *group differences >80% for Bayesian analysis.
Extended Data Fig. 6 LPC and LPA levels in blood plasma and LPA concentrations in the CSF of fasted AgRPDTR and WT.
a. Blood plasma concentrations of total LPCs and 18:0 LPC in fasted AgRPDTR animals (n = 5 fasted WT and n = 8 fasted AgRPDTR mice, group differences 88.1% for total LPC and 86,8% for LPC 18:1, Bayesian analysis). b. Blood plasma levels of different LPA subtypes in fasted WT and fasted AgRPDTR animals (n = 6 for LPA 16:0, 18:0 and n = 7 for 18:2, 18:3 and 20:4 for fasted WT; n = 8 for LPA 18:0 and 7 for all other LPA subtypes for fasted AgRPDTR animals, group differences 79.7% for LPA 16:0, 84.4% for LPA 18:0, 68.4% for LPA 18:2, 85.7% for LPA 18:3, 55.1% for LPA 20:4, Bayesian analysis). c. CSF levels of different LPA subtypes in fasted WT and fasted AgRPDTR mice (n = 6 for LPA 18:2 and 7 for all other LPAs in fasted WT and n = 5 for fasted AgRPDTR mice, group differences 68.8% for LPA 16:0, 69.4% for LPA 18:0, 97.3% for LPA 18:2, 95.5% for LPA 20:4, Bayesian analysis). d. Individual CSF LPA levels displayed for corresponding animals show high variance (n = 6 for LPA 18:2 and 7 for all other LPAs in fasted WT and n = 5 for fasted AgRPDTR mice). e. Ratio calculated for different LPA subtypes to total LPA levels (n = 6 for LPA 18:2 and 7 for all other LPAs in fasted WT and n = 5 for fasted AgRPDTR mice, group differences for LPA 16:0 90.2%, for LPA 18:0 97.5%, for LPA 18:1 93.5%, for LPA 18:2 98.8%, for LPA 20:4 70.5%, Bayesian analysis). Whiskers represent min to max, box extends from the 25th to the 75th percentile, middle line represents the median. Points represent individual values. Group differences of * >80% or **>90% are shown for Bayesian analysis.
Extended Data Fig. 7 Expression of synaptic lipid modulating molecules ATX and PRG-1 in the prefrontal cortex.
a. Nissl stained coronal section of the prefrontal cortex reaching up to the medial prefrontal cortex (mPFC, ACA anterior cingular area) and corresponding to the coronal levels of the in-situ hybridizations shown for ATX and PRG-1. Scale bar: 800 µm b. ATX expression is predominantly found in the upper prefrontal cortex layers (especially in layers II/III). Data was validated in three experiments. c. PRG-1 is strongly expressed in the layers II/III of the prefrontal cortex but is also found at high expression levels in the layer IV. Data was validated in two experiments. Images are adapted from the Allen Institute for Brain Science (2004). Allen Mouse Brain Atlas [70613967 for Atx and 73992941 for Prg-1] (mouse.brain-map.org).
Supplementary information
Supplementary Information
Supplementary methods for the Extended Data files and detailed description of Bayesian statistical methods, and references.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
About this article
Cite this article
Endle, H., Horta, G., Stutz, B. et al. AgRP neurons control feeding behaviour at cortical synapses via peripherally derived lysophospholipids. Nat Metab 4, 683–692 (2022). https://doi.org/10.1038/s42255-022-00589-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00589-7