Abstract
Efferocytosis, the clearance of apoptotic cells (ACs) by macrophages, is critical for tissue resolution, with defects driving many diseases. Mechanisms of efferocytosis-mediated resolution are incompletely understood. Here, we show that AC-derived methionine regulates resolution through epigenetic repression of the extracellular signal-regulated kinase 1/2 (ERK1/2) phosphatase Dusp4. We focus on two key efferocytosis-induced pro-resolving mediators, prostaglandin E2 (PGE2) and transforming growth factor beta 1 (TGF-β1), and show that efferocytosis induces prostaglandin-endoperoxide synthase 2/cyclooxygenase 2 (Ptgs2/COX2), leading to PGE2 synthesis and PGE2-mediated induction of TGF-β1. ERK1/2 phosphorylation/activation by AC-activated CD36 is necessary for Ptgs2 induction, but this is insufficient owing to an ERK−DUSP4 negative feedback pathway that lowers phospho-ERK. However, subsequent AC engulfment and phagolysosomal degradation lead to Dusp4 repression, enabling enhanced p-ERK and induction of the Ptgs2−PGE2−TGF-β1 pathway. Mechanistically, AC-derived methionine is converted to S-adenosylmethionine, which is used by DNA methyltransferase-3A (DNMT3A) to methylate Dusp4. Bone-marrow DNMT3A deletion in mice blocks COX2/PGE2, TGF-β1, and resolution in sterile peritonitis, apoptosis-induced thymus injury and atherosclerosis. Knowledge of how macrophages use AC-cargo and epigenetics to induce resolution provides mechanistic insight and therapeutic options for diseases driven by impaired resolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
This study did not generate any unique datasets or codes. All other data can be made available from the authors on reasonable request. Additional data associated with the paper can be found in the Supplementary Information. Source data are provided with this paper.
References
Dalli, J. & Serhan, C. N. Pro-resolving mediators in regulating and conferring macrophage function. Front. Immunol. 8, 1400 (2017).
Doran, A. C., Yurdagul, A. Jr & Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol. 20, 254–267 (2020).
Morioka, S., Maueroder, C. & Ravichandran, K. S. Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity 50, 1149–1162 (2019).
Linton, M. F. et al. Macrophage apoptosis and efferocytosis in the pathogenesis of atherosclerosis. Circ. J. 80, 2259–2268 (2016).
Kojima, Y., Weissman, I. L. & Leeper, N. J. The role of efferocytosis in atherosclerosis. Circulation 135, 476–489 (2017).
Yurdagul, A. Jr et al. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab. 31, 518–533 e510 (2020).
Zhang, S. et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 29, 443–456 e445 (2019).
A-Gonzalez, N. et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31, 245–258 (2009).
Fadok, V. A. et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101, 890–898 (1998).
Nakanishi, M. & Rosenberg, D. W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 35, 123–137 (2013).
Funk, C. D. & FitzGerald, G. A. COX-2 inhibitors and cardiovascular risk. J. Cardiovasc. Pharmacol. 50, 470–479 (2007).
Cheng, H., Huang, H., Guo, Z., Chang, Y. & Li, Z. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics 11, 8836–8854 (2021).
Tang, E. H., Libby, P., Vanhoutte, P. M. & Xu, A. Anti-inflammation therapy by activation of prostaglandin EP4 receptor in cardiovascular and other inflammatory diseases. J. Cardiovasc. Pharmacol. 59, 116–123 (2012).
Takayama, K. et al. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J. Biol. Chem. 277, 44147–44154 (2002).
Gitlin, J. M. & Loftin, C. D. Cyclooxygenase-2 inhibition increases lipopolysaccharide-induced atherosclerosis in mice. Cardiovasc. Res. 81, 400–407 (2009).
Kirkby, N. S. et al. COX-2 protects against atherosclerosis independently of local vascular prostacyclin: identification of COX-2 associated pathways implicate Rgl1 and lymphocyte networks. PLoS ONE 9, e98165 (2014).
Yu, Z. et al. Disruption of the 5-lipoxygenase pathway attenuates atherogenesis consequent to COX-2 deletion in mice. Proc. Natl Acad. Sci. USA 109, 6727–6732 (2012).
Narasimha, A. et al. A novel anti-atherogenic role for COX-2–potential mechanism for the cardiovascular side effects of COX-2 inhibitors. Prostaglandins Other Lipid Mediat. 84, 24–33 (2007).
Yoshimura, A., Wakabayashi, Y. & Mori, T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J. Biochem. 147, 781–792 (2010).
Mallat, Z. et al. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 89, 930–934 (2001).
Martinez, J. et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl Acad. Sci. USA 108, 17396–17401 (2011).
Mao, Y. et al. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin. Cancer Res. 20, 4096–4106 (2014).
Sun, W. H. et al. Induction of cyclooxygenase-2 in rat gastric mucosa by rebamipide, a mucoprotective agent. J. Pharmacol. Exp. Ther. 295, 447–452 (2000).
Mato, J. M., Martinez-Chantar, M. L. & Lu, S. C. S-adenosylmethionine metabolism and liver disease. Ann. Hepatol. 12, 183–189 (2013).
Parkhitko, A. A., Jouandin, P., Mohr, S. E. & Perrimon, N. Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. Aging Cell 18, e13034 (2019).
Quinlan, C. L. et al. Targeting S-adenosylmethionine biosynthesis with a novel allosteric inhibitor of Mat2A. Nat. Chem. Biol. 13, 785–792 (2017).
Finkelstein, J. D. Pathways and regulation of homocysteine metabolism in mammals. Semin. Thromb. Hemost. 26, 219–225 (2000).
Okano, M., Bell, D. W., Haber, D. A. & Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247–257 (1999).
Cho, K. N. et al. Prostaglandin E2 induces MUC8 gene expression via a mechanism involving ERK MAPK/RSK1/cAMP response element binding protein activation in human airway epithelial cells. J. Biol. Chem. 280, 6676–6681 (2005).
Xia, Q. et al. Induction of COX-2-PGE2 synthesis by activation of the MAPK/ERK pathway contributes to neuronal death triggered by TDP-43-depleted microglia. Cell Death Dis. 6, e1702 (2015).
Xiong, W., Frasch, S. C., Thomas, S. M., Bratton, D. L. & Henson, P. M. Induction of TGF-beta1 synthesis by macrophages in response to apoptotic cells requires activation of the scavenger receptor CD36. PLoS ONE 8, e72772 (2013).
Cai, B. et al. Macrophage MerTK promotes liver fibrosis in nonalcoholic steatohepatitis. Cell Metab. 31, 406–421 e407 (2020).
Gerlach, B. D. et al. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab. 33, 2445–2463 e2448 (2021).
Nishi, C., Yanagihashi, Y., Segawa, K. & Nagata, S. MERTK tyrosine kinase receptor together with TIM4 phosphatidylserine receptor mediates distinct signal transduction pathways for efferocytosis and cell proliferation. J. Biol. Chem. 294, 7221–7230 (2019).
Lang, R., Hammer, M. & Mages, J. DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. J. Immunol. 177, 7497–7504 (2006).
Newson, J. et al. Inflammatory resolution triggers a prolonged phase of immune suppression through COX-1/mPGES-1-derived prostaglandin E2. Cell Rep. 20, 3162–3175 (2017).
Cai, B. et al. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc. Natl Acad. Sci. USA 113, 6526–6531 (2016).
Ren, Y. & Savill, J. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J. Immunol. 154, 2366–2374 (1995).
Tse, K. & Ley, K. Transforming growth factor-beta: transforming plaque to stability. Eur. Heart J. 34, 3684–3686 (2013).
Takeuch, O. & Akira, S. Epigenetic control of macrophage polarization. Eur. J. Immunol. 41, 2490–2493 (2011).
Roy, D. G. et al. Methionine metabolism shapes T helper cell responses through regulation of epigenetic reprogramming. Cell Metab. 31, 250–266.e259 (2020).
Ji, J. et al. Methionine attenuates lipopolysaccharide-induced inflammatory responses via DNA methylation in macrophages. ACS Omega 4, 2331–2336 (2019).
Shikauchi, Y. et al. SALL3 interacts with DNMT3A and shows the ability to inhibit CpG island methylation in hepatocellular carcinoma. Mol. Cell. Biol. 29, 1944–1958 (2009).
Guo, X. et al. Structural insight into autoinhibition and histone H3-induced activation of DNMT3A. Nature 517, 640–644 (2015).
Dai, Z., Mentch, S. J., Gao, X., Nichenametla, S. N. & Locasale, J. W. Methionine metabolism influences genomic architecture and gene expression through H3K4me3 peak width. Nat. Commun. 9, 1955 (2018).
Fredman, G. et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun. 7, 12859 (2016).
Merched, A. J., Ko, K., Gotlinger, K. H., Serhan, C. N. & Chan, L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 22, 3595–3606 (2008).
Takayama, K., Sukhova, G. K., Chin, M. T. & Libby, P. A novel prostaglandin E receptor 4-associated protein participates in antiinflammatory signaling. Circ. Res. 98, 499–504 (2006).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Sharma, M. et al. Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression. Circ. Res. 127, 335–353 (2020).
Toussirot, E., Bonnefoy, F., Vauchy, C., Perruche, S. & Saas, P. Mini-Review: The administration of apoptotic cells for treating rheumatoid arthritis: current knowledge and clinical perspectives. Front. Immunol. 12, 630170 (2021).
Mevorach, D. et al. Single infusion of donor mononuclear early apoptotic cells as prophylaxis for graft-versus-host disease in myeloablative HLA-matched allogeneic bone marrow transplantation: a phase I/IIa clinical trial. Biol. Blood Marrow Transpl. 20, 58–65 (2014).
Zhang, Z. et al. Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. EBioMedicine 45, 341–350 (2019).
Pujol-Autonell, I. et al. Efferocytosis promotes suppressive effects on dendritic cells through prostaglandin E2 production in the context of autoimmunity. PLoS ONE 8, e63296 (2013).
Fourgeaud, L. et al. TAM receptors regulate multiple features of microglial physiology. Nature 532, 240–244 (2016).
Kasikara, C. et al. Deficiency of macrophage PHACTR1 impairs efferocytosis and promotes atherosclerotic plaque necrosis. J. Clin. Invest. e145275 (2021).
Acknowledgements
We thank S. Mukherjee and A. Burke (Columbia University) for the Dnmt3afl/fl Vav1Cre+/– mice; R. Bowman and R. Levine (Memorial Sloan Kettering Cancer Center) for initial discussions about the experimental approach using Dnmt3a-targeted mice; and J. Wang and X. Huang (Columbia) for initial discussions on DNA methylation assays. We acknowledge C. Lu of the Columbia Center for Translational Immunology Core Facility for assisting in the flow cytometry and immunofluorescent imaging experiments, which were conducted in the Columbia Center for Translational Immunology Core Facility, funded by NIH grants P30CA013696, S10OD020056 and S10RR027050. This study was funded by a Transatlantic Network of Excellence (TNE-18CVD04) grant from the Leducq Foundation (to A.R.T. and I.T.) and by the following NIH grants: R00 DK115778 (to B.C.); T32 5T32HL007343-42 (to B.D.G.); K99 HL145131 (to A.Y.); R01 HL127464 (to I.T.); and R01 HL087123 and R35 HL145228 (to I.T.). B.C. received funding support from R00DK115778. S.S. and Y.S. acknowledge the Leukemia Research Foundation (Hollis Brownstein New Investigator Research Grant); AFAR (Sagol Network GerOmic Award); the Einstein Nathan Shock Center for the Biology of Aging, Deerfield (Xseed award); NIH P30 grant CA01333047; shared instrument grant NIH 1 S10 OD030286-01; and NIGMS grant 5 R01GM129350-04 (PI: Brenowitz). This work has also been supported in part by the Proteomics & Metabolomics Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292).
Author information
Authors and Affiliations
Contributions
P.B.A. and I.T. developed the study concept and experimental design. Key advice was provided by A.R.T., P.B.A., B.C. and S.S. P.B.A., S.R.S. and B.D.G. conducted the experiments. X.W., A.Y. and G.K. assisted with bone marrow transplants, Zymosan A1-induced peritonitis experiments, dexamethasone-induced thymocyte apoptosis experiments and atherosclerosis experiments. L.N.F.D. carried out analysis of intracellular 13C515N-methionine and 13C515N-SAM in macrophages under the guidance of J.M.K. Y.S. conducted the experiments to detect 13C-labelled DNA in macrophages under the guidance of S.S. P.B.A. and I.T. wrote the manuscript and the other co-authors provided comments and revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Derek W. Gilroy, Xiaoling Li and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Isabella Samuelson.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
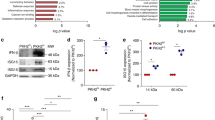
Extended Data Fig. 1 Additional experiments documenting the efferocytosis-Ptgs2/COX2-TGFβ1 pathway in macrophages. Related to Fig. 1.
BMDMs were incubated ± ACs, after which noninternalized ACs were removed by rinsing. The cells were assayed for Ptgs2 mRNA after an additional 1 h incubation and COX2 protein after 3 h (a); PGE2 in the media after 3 h (b); and TGFβ1 in the media after 18 h (c). For (d), experiments similar to those in panel a were analyzed for mouse and human Ptgs2/PTGS2 or Tgfb1/TGFβ1 mRNA to prove that mRNA being measured is not residual human mRNA derived from human apoptotic Jurkat cells. e, BMDMs were transfected with scrambled RNA (Scr) or siRbcn and then, after 72 h, assayed for Rbcn mRNA. f, BMDMs were transfected with Scr or siRbcn or treated with vehicle or bafilomycin A1 and then incubated with PKH26-labelled ACs for 45 min, followed by rinsing and quantification of percent PKH26+ macrophages of total macrophages. g, BMDMs were transfected with Scr or siPtgs2 and then, after 72 h, assayed for Ptgs2 mRNA. h, BMDMs were incubated ± ACs for 45 min, after which noninternalized ACs were removed by rinsing. The cells were assayed for Ptges mRNA after an additional 1 h incubation. i, BMDMs were transfected with scrambled RNA (Scr) or siPtges and then, after 72 h, assayed for Ptges mRNA. j-k, BMDMs were transfected with Scr, siPtger4, or siPtger2 and then, after 72 h, assayed for Ptger4 or Ptger2 mRNA, respectively. l, BMDMs were transfected with Scr or siTgfb1. After 72 h, the cells were incubated ± ACs for 45 min, after which noninternalized ACs were removed by rinsing. After an additional 1 h of incubation, the cells were assayed for Ptgs2 mRNA. m, BMDMs were transfected with Scr or siTgfb1 and then, after 72 h, assayed for Tgfb1 mRNA. All mRNA data are expressed relative to the first control group. Values are means ± SEM. ns, not significant (P > 0.05); n = 3 biological replicates. Two-sided P values were determined by a Student’s t-test for two groups or one-way ANOVA with Fisher’s LSD posthoc analysis for three or more groups.
Extended Data Fig. 2 Additional experiments documenting the role of SAM in the efferocytosis-Ptgs2/COX2-TGFβ1 pathway. Related to Figs. 2 & 3.
All AC incubations were 45 min, and Ptgs2 or Tgfb1 were assayed 1 or 6 h after AC removal, respectively. a, BMDMs pretreated 2 h with vehicle or the MAT2A inhibitor PF9366 were incubated with pHrodo-labeled ACs and quantified for the percent pHrodo-AC+ macrophages. Scale bar, 50 μm. b-c, BMDMs treated with scrambled RNA (Scr) or siMat2a were incubated ± ACs and then assayed for Ptgs2 orTgfb1. d, BMDMs treated with Scr or siMat2a were assayed for Mat2a. e, BMDMs treated with vehicle or SAM were assayed for SAM content. f, Jurkat cells were cultured in methionine-free DMEM supplemented with dialyzed FBS containing 13C515N-methionine and the MAT2A inhibitor, PF9366. The cells were rendered apoptotic after the third day of culture and analyzed by LC-MS/MS to show accumulation of 13C515N-methionine in the presence of MAT2A inhibitor, expressed as peak height, BMDMs cultured in methionine-free media with D-FBS and pretreated for 2 h with vehicle or bafilomycin A1 (Baf) were incubated 1 h with PKH26-labeled ACs whose proteins were labeled with 13C515N-methionine. AC+ and AC− macrophages were sorted and assayed for SAM content (g) and percent 13C515N-SAM of total SAM (h). i, Control or DNMT3A-KO BMDMs were incubated with PKH26−labelled ACs and then quantified for the percent PKH26-AC+ macrophages. Scale bar, 50 μm. j, Control and DNMT3A-KO BMDMs were immunoblotted for DNMT3A and β-actin. k, HMDMs treated with Scr or siDNMT3A were assayed for DNMT3A. l, BMDMs were incubated with IgG-coated RBCs for 45 min and then assayed 3 h later for COX2 MFI by flow cytometry. m-n, Control and DNMT3A-KO BMDMs treated with LPS or LPS + IFNγ for 4 h were assayed for Il6, Ptgs2, or COX2 (n). o-p, BMDMs treated with Scr or siDnmt3a were incubated for 24 h with vehicle and LPS + IFNγ or IL4 and assayed for Nos2 or Arg1. q, Control and DNMT3A-KO BMDMs were incubated ± ACs for 45 min and then assayed for SAM. r, BMDMs pretreated for 2 h with vehicle or bafilomycin A1 were incubated ± ACs whose proteins were labeled with 13C515N-methionine and then assayed 1 h after AC removal for 13C5-mC in DNA. s, BMDMs treated with Scr or siCreb1 were assayed for Creb1. All mRNA data are expressed relative to the first control group. Values are means ± SEM. n.s., not significant (P > 0.05); n = 3 biological replicates for all bar graphs except i (n = 6). Two-sided P values were determined by the Student’s t-test for two groups or one-way ANOVA with Fisher’s LSD posthoc analysis for three or more groups.
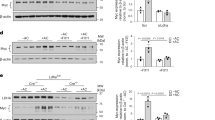
Extended Data Fig. 3 Experiments documenting the role of ERK, CD36, and DUSP4 in the efferocytosis-Ptgs2/COX2-TGFβ1 pathway. Related to Fig. 4.
AC incubations were 45 min, and Ptgs2 or Tgfb1 were assayed 1 or 6 h after AC removal, respectively. a, BMDMs incubated ± ACs were immunoblotted for p-ERK1/2, ERK1/2, and β-actin. b, BMDMs pretreated with vehicle or bafilomycin A1 were incubated ± PKH26-labelled ACs for 45 min and assayed by flow cytometry for p-ERK1/2 in PKH26+ (AC+) and PKH26- (AC−) macrophages. c-d, BMDMs transfected with scrambled RNA (Scr) or siMapk1 and siMapk3 were incubated ± ACs for 45 min and immunoblotted for ERK1/2, COX2, and β-actin or assayed for Ptgs2 or Tgfb1. e, WT or MerTK-KO BMDMs were incubated with PKH26-labelled ACs and assayed by flow cytometry for p-ERK1/2 or, after a 3-h chase, COX2. (f) BMDMs treated with Scr or siCd36 were assayed for Cd36. (g) BMDMs treated with Scr or siCd36 were incubated with pHrodo−labelled ACs and, after an 18-h chase, assayed by flow cytometry for TGFβ1 in pHrodo+ (AC+) and pHrodo- (AC−) macrophages. h, BMDMs pretreated for 2 h with vehicle or U0126 (MEK inhibitor) were incubated ± ACs and assayed for Dusp4. i, BMDMs treated with Scr, siDnmt3a, siDusp4, or siDnmt3a + siDusp4 were assayed for Dnmt3a or Dusp4. j-k, Control or DNMT3A-KO BMDMs transfected with Scr or siDusp4 as indicated were incubated ± ACs and assayed for Ptgs2 or Tgfb1. l-m, Control or DNMT3A-KO BMDMs treated with Scr or siDusp1 as indicated were incubated ± ACs and assayed for Ptgs2 or Tgfb1. n-o, BMDMs treated with Scr, siMat2a, siDusp4, or siMat2a + siDusp4 were incubated ± ACs and assayed for Tgfb1. p-q, BMDMs treated with Scr, siMapk1/3, siDusp4, or siMapk1/3 + siDusp4 were incubated ± ACs and assayed for Tgfb1, Mapk3, Mapk1, and Dusp4 after a 6-h chase. Data are expressed relative to the first control group. Values are means ± SEM. n.s., not significant (P > 0.05); n = 3 biological replicates. Two-sided P values were determined by the Student’s t-test for two groups or one-way ANOVA with Fisher’s LSD posthoc analysis for three or more groups.
Extended Data Fig. 4 In vivo evidence of AC−induced COX2-TGFβ1 pathway. Related to Fig. 5.
Wild-type (WT) C57BL/6 J mice were transplanted with bone marrow from Vav1Cre+/– (Control) or Dnmt3afl/fl Vav1Cre+/– (H-DNMT3A-KO) mice and, after 4 weeks, injected with PBS or dexamethasone (DEX). After 4 h, the thymus was harvested and immunostained with anti-annexin V to document the initial increase in apoptosis after PBS or DEX injection, n = 4 mice per group (a). For b (left), thymi were immunostained with Mac2 (green) and DNMT3A (Red). White arrows indicate non-macrophage DNMT3A, and brown arrows indicate macrophage DNMT3A. For b (right), documentation of co-localized p-ERK1/2, COX2, and Mac2. Representative image from n = 4 mice per group. Scale bar, 50 μm. c-d, Wildtype (WT) C57BL/6 J mice transplanted with bone marrow from control or H-DNMT3A-KO mice were injected i.p. with 1 mg/mL Zymosan A1. After 12 h, peritoneal exudate cells were analyzed by flow cytometry for F4/80+ and COX2+ cells (n = 4 mice/group) LAP-TGFβ1+ cells (n = 4 mice/group). e-m, Ldlr-/- (LDLR-KO) mice were transplanted with bone marrow from control or H-DNMT3A-KO mice and, after 4 weeks, fed a western-type diet (WD) for 12 weeks. Aortic root sections were immunostained for Mac2 and DNMT3A. Brown arrows indicate macrophage DNMT3A. Representative image from n = 8 mice per group (e); body weight, n = 10 mice/group (f); and the plasma or blood was assayed for the indicated metabolic and immune cell parameters, n = 10 mice/group (g-l) and the lipoprotein-cholesterol profile by FPLC (m). Scale bar, 50 μm. Values are means ± SEM; n.s., not significant (P > 0.05). Two-sided P values were determined by the Student’s t-test for two groups or one-way ANOVA with Fisher’s LSD posthoc analysis for three or more groups.
Extended Data Fig. 5 DNMT3A mediates efferocytosis and resolution in vivo. Related to Fig. 6.
BMDMs were pretreated with vehicle or a TGFβ1R inhibitor for 2 h and with vehicle or recombinant TGFβ1 for 1 h, as indicated. The macrophages were then incubated with PKH26−labelled ACs for 45 mins, followed by rinsing and quantification of percent PKH26-AC+ macrophages of total macrophages, n = 4 biological replicates. b-f, Wildtype (WT) C57BL/6 J mice were transplanted with bone marrow from control or H-DNMT3A-KO mice and, after 4 weeks, injected with PBS or dexamethasone (DEX). After 18 h, the thymi were weighed, n = 7 and 9 mice for PBS and DEX groups respectively (b); immunostained for DAPI, TUNEL, and Mac2 (c); assayed for F4/80+ cells, n = 5 mice per group (d); and assayed for TNF-a, n = 3 and 5 mice for PBS and DEX groups respectively and IL-6 by ELISA, n = 3 and 4 mice for PBS and DEX groups respectively (e-f). The image in b illustrates thymic macrophages with cytoplasmic TUNEL as an example of efferocytosing thymic macrophages. Scale bar, 100 μm. g, The peritoneal exudates were assayed for Ly6G+ polymorphonuclear cells (PMN) 18 hours after Zymosan A1 injection, n = 3 mice per group. h, Wild-type mice received 200 ng/mL recombinant TGFβ1 i.p. or vehicle control 15 and 20 hours after Zymosan injection and then assayed for the number of PMNs 4 hours later, n = 3 mice/group. i, Ldlr-/- (LDLR-KO) mice were transplanted with bone marrow from control or H-DNMT3A-KO mice and, after 4 weeks, fed a western-type diet (WD) for 12 weeks. Aortic root sections were quantified for lesional area, n = 8 mice/group. Values are means ± SEM; n.s., not significant (P > 0.05). Two-sided P values were determined by the Student’s t-test for two groups or one-way ANOVA with Fisher’s LSD posthoc analysis for three or more groups.
Supplementary information
Supplementary Information
Supplementary Tables 1–3 and Extended Data figure legends.
Source data
Source Data Fig. 3
Unprocessed western blots for Fig. 3b and 3m.
Source Data Extended Data Fig. 1
Unprocessed western blots for Extended Data Fig1a.
Source Data Extended Data Fig. 2
Unprocessed western blots for Extended Data Fig. 2j and 2n.
Source Data Extended Data Fig. 3
Unprocessed western blots for Extended Data Fig. 3a and 3c.
Rights and permissions
About this article
Cite this article
Ampomah, P.B., Cai, B., Sukka, S.R. et al. Macrophages use apoptotic cell-derived methionine and DNMT3A during efferocytosis to promote tissue resolution. Nat Metab 4, 444–457 (2022). https://doi.org/10.1038/s42255-022-00551-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00551-7
This article is cited by
-
MSCs mediate long-term efficacy in a Crohn’s disease model by sustained anti-inflammatory macrophage programming via efferocytosis
npj Regenerative Medicine (2024)
-
Dysregulated cellular metabolism in atherosclerosis: mediators and therapeutic opportunities
Nature Metabolism (2024)
-
epHero – a tandem-fluorescent probe to track the fate of apoptotic cells during efferocytosis
Cell Death Discovery (2024)
-
Specialized pro-resolving mediators in vascular inflammation and atherosclerotic cardiovascular disease
Nature Reviews Cardiology (2024)
-
Cystathionine gamma-lyase (Cth) induces efferocytosis in macrophages via ERK1/2 to modulate intestinal barrier repair
Cell Communication and Signaling (2023)