Abstract
Severe cases of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are associated with elevated blood glucose levels and metabolic complications. However, the molecular mechanisms for how SARS-CoV-2 infection alters glycometabolic control are incompletely understood. Here, we connect the circulating protein GP73 with enhanced hepatic gluconeogenesis during SARS-CoV-2 infection. We first demonstrate that GP73 secretion is induced in multiple tissues upon fasting and that GP73 stimulates hepatic gluconeogenesis through the cAMP/PKA signaling pathway. We further show that GP73 secretion is increased in cultured cells infected with SARS-CoV-2, after overexpression of SARS-CoV-2 nucleocapsid and spike proteins and in lungs and livers of mice infected with a mouse-adapted SARS-CoV-2 strain. GP73 blockade with an antibody inhibits excessive glucogenesis stimulated by SARS-CoV-2 in vitro and lowers elevated fasting blood glucose levels in infected mice. In patients with COVID-19, plasma GP73 levels are elevated and positively correlate with blood glucose levels. Our data suggest that GP73 is a glucogenic hormone that likely contributes to SARS-CoV-2-induced abnormalities in systemic glucose metabolism.
Similar content being viewed by others
Main
Under physiological conditions, blood glucose levels are maintained within a narrow range to prevent hypoglycemia during fasting and excessive hyperglycemia following intake of a high-carbohydrate meal1. Glucose homeostasis is achieved primarily via hormonal modulation of glucose production by the liver2 and glucose uptake by skeletal muscle, heart muscle and adipose tissue3,4. Hepatic glucose production (HGP) involves a combination of glycogen breakdown (glycogenolysis) and de novo synthesis of glucose from noncarbohydrate precursors (gluconeogenesis)2. Gluconeogenesis is the main contributor to hepatic and renal glucose production during prolonged fasting states5. The rate of gluconeogenic flux is controlled by the activities of key unidirectional enzymes, such as pyruvate carboxylase (PCX), phosphoenolpyruvate carboxykinase 1 (PCK1), fructose 1,6-bisphosphatase (FBP1) and glucose-6-phosphatase (G6Pase)6. The genes encoding these proteins are strongly controlled at the transcriptional level by key hormones, including insulin, glucagon and glucocorticoids7. Excessive HGP not only contributes to exaggerated fasting and postprandial hyperglycemia in patients with type 1 diabetes (T1D) and type 2 diabetes (T2D) but also contributes to stress-, infection- and inflammation-associated hyperglycemia8,9.
Since January 2020, the world has been facing an unprecedented outbreak of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2. Recent clinical data have suggested a close interaction between COVID-19 and diabetes10,11,12,13,14. Preexisting diabetes substantially increases COVID-19 mortality and morbidity. An increase in new-onset hyperglycemia, diabetic ketoacidosis (DKA) and diabetes in patients with COVID-19 is commonly observed. Although impaired insulin secretion due to β-cell infection could plausibly contribute to the metabolic dysregulation observed in patients with COVID-19 (refs. 15,16,17), the pathogenic mechanisms underlying new-onset T2D are largely unknown.
GP73 is a type II transmembrane Golgi protein located at the luminal surface of the Golgi apparatus, consisting of a short N-terminal cytoplasmic domain, a transmembrane domain and a large C-terminal domain18. GP73 cycles out of the cis-Golgi to endosomes for cleavage by the convertase furin, resulting in its release into the extracellular space19,20. Circulating GP73 is implicated in the regulation of cell-to-cell communication triggered by the unfolded protein response21. The present study provides evidence that circulating GP73 promotes HGP in endocrine and autocrine manners. Notably, plasma GP73 levels are elevated in patients with COVID-19 and GP73 is necessary for excessive glucogenesis stimulated by SARS-CoV-2 infection. Therefore, targeting GP73 might be a potential therapeutic strategy for patients presenting alterations in the levels of this hormone.
Results
GP73 is induced from multiple tissues upon fasting and high-fat diet
A previous report showed that GP73 is cleaved at the R52VRR55 proprotein convertase (PC) consensus site by the protease furin, resulting in its release into the extracellular space19,20. The elevated serum GP73 levels in patients with nonalcoholic steatohepatitis (NASH) prompted us to examine the potential metabolic function of circulating GP73 (ref. 22). We first monitored to investigate whether circulating GP73 levels change in response to nutrient fluctuations. Indeed, fasting in humans and mice increased plasma GP73 levels (Fig. 1a,b). As intracellular GP73 overexpression led to the release of GP73 into the extracellular space (Extended Data Fig. 1a,b), we examined and compared GP73 expression in metabolically important organs isolated from ad libitum-fed and fasted mice to delineate the source of GP73 under fasting conditions. After 24 h of fasting, a strong upregulation of GP73 messenger RNA expression in the heart, liver, kidney, white adipose tissue (WAT) and pancreas was observed (Fig. 1c). Consistent with this finding, the intensity of GP73 immunofluorescence staining in liver, WAT and pancreatic tissues was significantly increased upon 24 h of fasting (Fig. 1d). GP73 secretion from cultured HepG2 cells was also promoted in response to the fasting-related signal forskolin (FSK; Fig. 1e). Fasting-related secretion of GP73 prompted us to examine circulating GP73 levels in the context of metabolic disturbance. Notably, plasma GP73 was markedly increased (approximately threefold) in mice fed a high-fat diet (HFD; Fig. 1f). In particular, HFDs induced dramatically increased GP73 mRNA expression in heart, liver and WAT tissues (Fig. 1g). Thus, GP73 secretion is induced from multiple tissues upon nutrient fluctuations.
a,b, Plasma GP73 levels in humans following an overnight fast (a; n = 7) or in mice fed ad libitum (ad-lib) or fasted for the indicated times (b; n = 4). P = 0.0005 for fasted versus fed by two-tailed paired Student’s t-tests (a), P = 0.0180 for fasted (24 h) versus ad-lib by one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test (b). c, GP73 mRNA expression in various organs from mice fed ad-lib or fasted for 24 h. BAT, brown adipose tissue; AG, adrenal gland (n = 3). P = 0.0004 for fasted heart versus ad-lib, P = 0.0031 for fasted liver versus ad-lib, P = 0.0012 for fasted kidney versus ad-lib, P = 0.0097 for fasted WAT versus ad-lib, P < 0.0001 for fasted pancreas versus ad-lib by two-tailed Student’s t-tests. d, Representative of three confocal immunofluorescence images of GP73 staining in liver, WAT and pancreatic sections from three mice fed ad libitum or fasted for 24 h. Green represents GP73 and blue represents the nucleus. e, Supernatant GP73 levels in HepG2 cells treated with FSK (20 µM) for the indicated times. P = 0.0018 for FSK versus PBS (48 h), P = 0.0218 for FSK versus PBS (72 h) by two-tailed Student’s t-tests. f,g, Plasma GP73 levels (f) or GP73 mRNA expression in various organs in mice fed a regular diet (reg) or HFD for 4 months (f, n = 4 or 5 in each group; g, n = 3). P < 0.0001 for HFD versus reg (f) by two-tailed Student’s t-tests. P = 0.0010 for fasted heart versus ad-lib, P = 0.0002 for fasted WAT versus ad-lib, P = 0.0135 for fasted liver versus ad-lib by two-tailed Student’s t-tests (g). Animal and cell-based studies were performed independently at least three biological replicates with comparable results. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
Increase in circulating GP73 elevates fasting blood glucose
To understand the acute response to elevated circulating GP73 levels, we expressed and purified the mouse GP73 C-terminal fragment (56–401; rmGP73) (Extended Data Fig. 2a,b). A mouse GP73 sandwich ELISA was developed and specificity was confirmed using plasma from wild-type (WT) and GP73 knockout (KO) mice (Extended Data Fig. 2c,d). Administration of a single dose of 0.1 mg kg−1 rmGP73 led to an approximately twofold increase in plasma GP73 levels compared to control mice, with a half-life of approximately 30 min (Extended Data Fig. 2e,f). We then experimentally elevated plasma GP73 levels via intravenous (i.v.) injection of 0.1 mg kg−1 rmGP73 into mice subjected to a preceding 12-h fast. In this setting, rmGP73 induced an immediate spike in blood glucose levels and compensatory hyperinsulinemia (Fig. 2a,b). A GP73-specific antibody completely blocked the glucose-stimulating effect of recombinant GP73 (Fig. 2c). Interestingly, GP73 treatment had no effect on plasma levels of glucagon, catecholamines and glucocorticoids, catabolic hormones known to induce hepatic glucose release (Extended Data Fig. 3a–d). We then injected rmGP73 daily for 3 d. Repeated GP73 injection produced a sharp rise in blood glucose levels (Fig. 2d).
a, Plasma glucose levels at the indicated times after a single dose of i.v. rmGP73 (0.1 mg kg−1) or PBS in mice subjected to a preceding 12 h fast (n = 6). P = 0.0047 for rmGP73 versus PBS (15 min), P = 0.0065 for rmGP73 versus PBS (30 min), P < 0.0001 for rmGP73 versus PBS (60 min), P = 0.0084 for rmGP73 versus PBS (90 min) and P = 0.0018 for rmGP73 versus PBS (120 min) by two-way ANOVA followed by Bonferroni’s post hoc test. b, Plasma insulin levels 15 min after rmGP73 injection (n = 6). P < 0.0001 for rmGP73 versus PBS (15 min) by two-tailed Student’s t-tests. c, Plasma glucose levels 15 min after rmGP73 injection with IgG isotype or anti-GP73 antibody (n = 5). P = 0.0027 for rmGP73 versus PBS (isotype), P = 0.0067 for anti-GP73 versus isotype (rmGP73) by one-way ANOVA followed by Bonferroni’s post hoc test. d, Plasma glucose levels at the indicated times after rmGP73 injection once daily for 3 d (n = 6). P = 0.0016 for first versus PBS, P < 0.0001 for second versus PBS, P = 0.0020 for third versus PBS by two-way ANOVA followed by Bonferroni’s post hoc test. e–h, ITT (e), intravenous glucose tolerance test (IGTT; f), PTT with or without anti-GP73 antibody (g) or alanine tolerance test (ATT; h) in mice 2 h after rmGP73 injection (n = 5 or 6). P = 0.6384 for rmGP73 versus PBS by two-way ANOVA test (e), P < 0.0001 for rmGP73 versus PBS (30 min and 60 min), P = 0.0012 for rmGP73 versus PBS (90 min), P = 0.0176 for rmGP73 versus PBS (120 min) by two-way ANOVA followed by Bonferroni’s post hoc test (f), P < 0.0001 for rmGP73 + anti-GP73 versus rmGP73 + isotype (15 min, 30 min, 60 min, 90 min and 120 min) (g), P = 0.0046 for rmGP73 versus PBS (0 min), P = 0.0126 for rmGP73 versus PBS (15 min), P = 0.0063 for rmGP73 versus PBS (30 min) (h) by two-way ANOVA followed by Bonferroni’s post hoc test. i, Plasma glucose levels 15 min after injection of IgG isotype or anti-GP73 antibody in mice subjected to a preceding 12 h fast (n = 5). P = 0.0036 for anti-GP73 versus isotype by two-tailed Student’s t-tests. j, Immunoblotting (IB) analysis of AKT phosphorylation (AKT-p) in PMHs, HepG2 or L6 cells treated with the indicated concentrations of insulin in the presence or absence of GP73. Animal and cell-based studies were performed independently at least three biological replicates with comparable results. Data are mean ± s.e.m. *P < 0.05,**P < 0.01, ***P < 0.001.
Hyperglycemia is caused either by impaired glucose clearance and/or excess glucose production through glycogenolysis or gluconeogenesis. To determine which of these processes caused GP73-induced hyperglycemia, we performed an insulin tolerance test (ITT). There were no significant differences in mice challenged with rmGP73 (Fig. 2e). However, when we assessed insulin resistance with the glucose tolerance test (GTT), we observed that rmGP73 severely impaired glucose clearance (Fig. 2f). The pyruvate tolerance test (PTT) indicated that the conversion of pyruvate to glucose was greatly enhanced after GP73 challenge in overnight-fasted, glycogen-depleted mice and that this effect was completely blocked by anti-GP73 treatment (Fig. 2g). GP73 elevation also stimulated alanine-driven gluconeogenesis in a glycogen-depleted state (Fig. 2h). No significant difference was found in hepatic glycogen content between the rmGP73- and GFP-injected mice (Extended Data Fig. 3e), suggesting that glycogenolysis was not affected by GP73. In particular, neutralizing circulating GP73 with a GP73 mAb in fasting animals attenuated the ability of mice to maintain normoglycemia (Fig. 2i). Notably, GP73 treatment tended to decrease insulin-stimulated phosphorylation of AKT at Ser473 in primary mouse hepatocytes (PMHs), HepG2 cells and L6 rat myotubes (Fig. 2j). Thus, an increase in circulating GP73 may elevate fasting glycemia mainly by potentiating endogenous glucose production and reducing glucose clearance (Extended Data Fig. 3f).
Circulating GP73 traffics to the liver to stimulate gluconeogenesis
To examine the primary target of circulating GP73, rmGP73 labeled with Cy7 (rmGP73-Cy7) was injected into mice and an in vitro imaging system (IVIS) was used to identify sites of accumulation. In contrast to free Cy7, rmGP73-Cy7 trafficked primarily to the liver and kidney (Extended Data Fig. 4a) and exhibited higher accumulation in these two tissues 30 min after injection (Fig. 3a and Extended Data Fig. 4b). In addition, the fluorescence signal in the liver 19 h after administration was 20% of the signal at 1 h (Extended Data Fig. 4c,d). Moreover, GP73 bound to the surface of hepatocytes in a dose-dependent and saturable manner (Fig. 3b and Extended Data Fig. 4e).
a, In vivo imaging of various organs from two mice 30 min after rmGP73-Cy7 or free Cy7 injection. Two representative images from four mice are shown. b, The level of biotin on the hepatocyte surface upon incubation of HepG2 cells with increasing concentrations of rhGP73-biotin. Specific binding (shown in red) was calculated as the difference between the two curves. c, Glucose production in PMHs treated with rmGP73 at the indicated concentrations for 2 h. P < 0.0001 for 4 nM versus 0 nM, 8 nM versus 0 nM, 16 nM versus 0 nM, 32 nM versus 0 nM and 64 nM versus 0 nM by one-way ANOVA followed by Bonferroni’s post hoc test. d, Glucose production in PMHs treated with 32 nM rmGP73 or glucagon in the presence of cAMPS-Rp (20 μM), H89 (20 μM), LY2409021 (2.5 μM), or insulin (10 μM) for 2 h. P = 0.0070 for rmGP73 + insulin versus rmGP73, P = 0.0062 for rmGP73 + LY2409021 versus rmGP73, P = 0.0004 for glucagon + insulin versus glucagon, P < 0.0001 for rmGP73 + H89 versus rmGP73, rmGP73 + cAMPS-Rp versus rmGP73, glucagon + insulin versus glucagon, glucagon + LY2409021 versus glucagon, glucagon + H89 versus glucagon and glucagon + cAMPS-Rp versus glucagon by one-way ANOVA followed by Bonferroni’s post hoc test. e, Gluconeogenesis gene expression in PMHs treated with 32 nM rmGP73 for 2 h. P = 0.0051 for rmGP73 versus PBS (Pcx), P < 0.0001 for rmGP73 versus PBS (Pck1 and G6pc) by two-tailed Student’s t-tests. f, Immunoblotting analysis of phosphorylated PKA-C subunit (PKA-C-α-p) levels and substrate (phosphoRRXT*T*-PKA substrate, RRXpS/T) levels in HepG2 cells treated with 32 nM rmGP73 or 10 μM IBMX for the indicated times. DMSO, dimethylsulfoxide. g, Glucose production in PMHs isolated from WT or KO mice treated with or without rmGP73 (32 nM) for 4 h. P = 0.0090 for KO versus WT, P < 0.0001 for KO + rmGP73 versus KO by one-way ANOVA followed by Bonferroni’s post hoc test. h–j, Basal and clamp HGP (h), glucose disposal rate (GDR) (i) and insulin levels (j) in GP73 KO mice infused with GFP or recombinant GP73 during the clamp study. P = 0.0194 for rmGP73 versus GFP (basal), P = 0.0026 for rmGP73 versus GFP (clamp) (h), P = 0.0047 for rmGP73 versus GFP (i) and P = 0.8818 for rmGP73 versus GFP (j) by two-tailed Student’s t-tests. Animal and cell-based studies were performed independently at least three biological replicates with comparable results. Data are mean ± s.e.m. NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
We then directly assessed the effect of GP73 on glucose production in isolated PMHs. Treatment of PMHs isolated from overnight-fasted mice with rmGP73 promoted HGP in a dose- and time-dependent manner (Fig. 3c and Extended Data Fig. 5a). A similar result was observed in rat primary hepatocytes treated with recombinant rat GP73 (rrGP73; Extended Data Fig. 5b). In particular, a GP73-specific antibody blocked GP73-mediated hepatocyte glucose release (Extended Data Fig. 5c). Small molecular inhibitors of PKA, such as cAMPS-Rp and H89, completely blocked GP73-mediated hepatocyte glucose release in a similar manner as glucagon (Fig. 3d). In addition, we found that insulin exerted some suppressive effect on GP73-mediated glucose release (Fig. 3d). While the glucagon receptor (GCGR) allosteric antagonist LY2409021 completely blocked the effects of glucagon, it also partially suppressed the ability of GP73 to stimulate hepatocyte glucose release (Fig. 3d). The addition of rmGP73 also led to increased expression of key gluconeogenic enzymes, intracellular cAMP levels and PKA kinase activity (Fig. 3e and Extended Data Fig. 5d,e). Phosphorylation of the PKA-C-α subunit and PKA substrate levels in hepatocytes was also significantly increased by GP73 (Fig. 3f). Notably, rmGP73 addition in primary hepatocytes isolated from overnight-fasted GP73 KO mice was sufficient to completely rescue the HGP deficiency displayed by these cells (Fig. 3g).
We next performed hyperinsulinemic-euglycemic clamp studies in GP73-deficient genetic background mice (GP73 KO) and reconstituted circulating GP73 by infusing recombinant protein (Fig. 3h–j and Extended Data Fig. 6a–f). We were able to achieve blood GP73 levels comparable to those in WT animals (Extended Data Fig. 6c). In this setting, we found increased HGP and decreased whole-body glucose disposal (insulin sensitivity) upon GP73 administration (Fig. 3h,i).
GP73 induces a drastic remodeling of the PKA kinase hub
To determine the full extent of the hepatic signaling effect of circulating GP73 at the cellular level in the absence of systemic factors that may exert compensatory effects, we performed global phosphoproteomics analysis in PMHs treated with PBS, rmGP73 or glucagon in vitro. A total of 183 and 229 phosphosites were identified in the GP73-treated and glucagon-treated samples, respectively (Fig. 4a). Of the top 30 phosphosites that were highly upregulated by GP73, over 75% were also affected by glucagon (Fig. 4b), indicating that these two proteins have overlapping functions at the signaling level. Among the potentially functional phosphosites regulated by GP73, Ser1588 of inositol triphosphate receptor-1 (Itpr1, also known as InsP3R-I) attracted our attention (Fig. 4c). This protein is a target of PKA and plays a crucial role in glucagon-stimulated hepatic gluconeogenesis. Other proteins significantly regulated by GP73 that are known to be involved in gluconeogenesis signaling included PHKA2S729, GNMTY221, SLC16A1S491, MLXS45, BADS155, CTNNB1S552, GPX1S7, CYP2E1S129 and HCFC1S1516 (Fig. 4c).
a, Overlapping numbers of significantly upregulated phosphopeptides and downregulated phosphopeptides in PMHs treated with rmGP73 (64 nM) or glucagon (3 μM) for 1 h. Localization probability ≥0.75 and fold-change ≥1.5 or fold-change ≤0.67 were declared significant. Significantly regulated phosphopeptides by GP73 are shown in blue and those regulated by glucagon are shown in red. b, Heat map of the top 30 highly upregulated phosphosites in PMHs treated with rmGP73. c, Network analysis of proteins involved in the glucogenesis signaling pathway in PMHs treated with rmGP73. Each of the phosphosites is color-coded based on fold change. Circular shapes show each protein that is upregulated in GP73-treated cells compared to PBS-treated cells. Lines indicate protein–protein interactions curated from databases of experimentally defined kinase–substrate relationships (STRING, confidence >0.7). d,e, Specific kinase–substrate motifs (d) or distribution of matching kinases (e) according to the phosphoproteomics data from the rmGP73-treated sample (P < 0.05) using MoMo (https://meme-suite.org/tools/momo) and Kinase Enrichment Analysis 2 (KEA2) based on one-tailed Fisher’s exact test. f, KEGG enriched pathway analysis of significantly regulated phosphopeptides in PMHs treated with rmGP73 (P < 0.05) using DAVID Bioinformatics Resources 6.8. mTOR, Mammalian target of rapamycin; FoxO, Forkhead Box O; VEGF, Vascular endothelial growth factor; ErbB, Epidermal growth factor family of receptor tyrosine kinases; GnRH, Gonadotropin-releasing hormone. The bar plot shows significantly dysregulated pathways and one-tailed Fisher’s exact test P values are shown on the x axis.
To investigate the mechanisms responsible for the phosphoproteome changes, kinase–substrate motifs were extracted and a clear shift toward PKA kinase activation following GP73 or glucagon stimulation was identified (Fig. 4d and Extended Data Fig. 7a). Pathway analysis of the phosphosites regulated by GP73 revealed pathways related to cell–cell adhesion, cell endocytosis, mRNA processing, gene transcription and cell cycle regulation (Extended Data Fig. 7b). Other substrate motifs of key kinases previously implicated in the regulation of gluconeogenesis and insulin resistance, including GSK3B, PRKG1, PTK2B and CaMK2A, were also dysregulated, which further explains the ability of GP73 to affect glucose production and insulin sensitivity (Fig. 4e). Additional enriched kinase motifs following GP73 stimulation included mitogen-activated kinase (MAPK) 3/8/9/10/13/14 and cyclin-dependent kinase (CDK)1/2 (Fig. 4e). Global kinase enrichment analysis following GP73 stimulation revealed that GP73 induced drastic remodeling of the PKA hub in a similar manner as glucagon (Extended Data Fig. 8a,b). At the signaling level, the mTOR pathway, FoxO pathway and insulin pathway were positively enriched in GP73-treated hepatocytes (Fig. 4f). Therefore, GP73 induces drastic remodeling of the PKA kinase hub and multiple signaling pathways.
SARS-CoV-2 infection promotes GP73 production and secretion
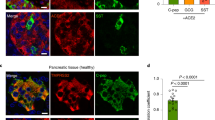
A proteomics analysis of patients with COVID-19 shows elevated expression of GP73 in the lung, liver and renal tissues23, indicating a possible link between SARS-CoV-2 infection and GP73 expression. To assess whether infection with this virus altered GP73 secretion, Huh-7 cells were infected with SARS-CoV-2 (Extended Data Fig. 9a–c) and GP73 secretion was examined over time during infection. Cultured hepatocytes exposed to SARS-CoV-2 exhibited a robust dose- and time-dependent increase in GP73 secretion (Fig. 5a,b). Intracellular GP73 protein expression was also increased in SARS-CoV-2-infected cells (Fig. 5c). To further evaluate this finding in a mouse model infected with a mouse-adapted strain at passage 6 (ref. 24) (MASCp6; Extended Data Fig. 9d), we examined and compared GP73 expression in multiple tissues from MASCp6- or mock-infected mice. On day 2 after infection, upregulation of GP73 expression in the lung and liver tissues was observed (Fig. 5d-e). Of note, the increased staining of GP73 occurred in lung cells stained positive with S-protein as well as in S-protein-negative neighboring cells, indicating that SARS-CoV-2 induced GP73 expression likely in both a direct and a paracrine manner (Extended Data Fig. 9e).
a,b, Supernatant GP73 levels in Huh-7 cells infected with SARS-CoV-2 at the indicated multiplicity of infection (MOI) for 24 h (a) or with SARS-CoV-2 (MOI 0.1) for the indicated times (b). P = 0.0177 for 0.01 MOI versus 0 MOI, P = 0.0002 for 0.1 MOI versus 0 MOI (a), P = 0.0049 for 0.1 MOI versus 0 MOI (24 h), P < 0.0001 for 0.1 MOI versus 0 MOI (48 h), P = 0.0010 for 0.1 MOI versus 0 MOI (72 h) by two-way ANOVA followed by Bonferroni’s post hoc test. c, Intracellular GP73 levels in Huh-7 cells infected with SARS-CoV-2 (CoV-2) at the indicated MOIs for 24 h. d, Representative of three confocal immunofluorescence images of GP73 staining in liver and lung sections from three MASCp6-infected mice. Green represents GP73 and blue represents the nucleus. e, GP73 mRNA expression in lung, liver and kidney tissues from mice inoculated with 1.6 × 104 plaque-forming units (p.f.u.) of MASCp6 and killed at day 2 after inoculation (n = 6). P = 0.0079 for infected versus mock (lung), P = 0.0389 for infected versus mock (liver) by two-tailed Student’s t-tests. f,g, Supernatant GP73 levels in Huh-7 cells transfected with plasmids expressing the indicated SARS-CoV-2 proteins (f) or different concentrations of Flag-N (g). P = 0.0097 for NSP12 versus vector, P = 0.0044 for NSP14 versus vector, P = 0.0012 for S versus vector and P = 0.0031 for N versus vector by two-tailed Student’s t-tests (f), P = 0.0036 for 0.5 μg versus 0 μg and P < 0.0001 for 4 μg versus 0 μg by one-way ANOVA followed by Bonferroni’s post hoc test (g). h, Supernatant GP73 levels in Huh-7 cells challenged with SARS-CoV-2pp or VSV-Gpp for the indicated times. P = 0.0205 for SARS-2-Spp versus VSV-Gpp (12 h), P = 0.0004 for SARS-2-Spp versus VSV-Gpp (24 h) and P = 0.0006 for SARS-2-Spp versus VSV-Gpp (48 h) by two-way ANOVA followed by Bonferroni’s post hoc test. i,j, Immunoprecipitation analysis of GP73 interaction with N (i) or S (j) protein in 293T cells transfected with Flag-N or Flag-S. Animal and cell-based studies were performed independently at least three biological replicates with comparable results. Data are mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001.
We then evaluated the effect of individual SARS-CoV-2 proteins on GP73 secretion. By screening 23 viral proteins, we found that the expression of the N and S proteins significantly promoted GP73 secretion, whereas the NSP12 and NSP14 proteins exhibited the opposite effects (Fig. 5f and Extended Data Fig. 9f). Notably, GP73 secretion was enhanced by the N protein in a dose-dependent manner (Fig. 5g and Extended Data Fig. 9g). HIV pseudotypes bearing SARS-2-S (SARS-CoV-2pp) also enhanced GP73 secretion when presented during infection compared to VSV-Gpp (Fig. 5h). Similar results were obtained in a panel of human cell lines (Extended Data Fig. 9h). Notably, both the N and S proteins coimmunoprecipitated with endogenous GP73 (Fig. 5i,j). Together, these results demonstrate that SARS-CoV-2 proteins regulate the secretion of GP73.
Plasma GP73 is pathologically elevated in patients with SARS-CoV-2 infection
We then examined plasma GP73 expression in patients with COVID-19. Notably, SARS-CoV-2-infected patients had approximately twofold higher circulating GP73 levels than the reference population (Supplementary Table 1 and Fig. 6a). When disease status was classified into mild, moderate and severe, the serum GP73 concentration was significantly higher in patients with severe or moderate disease than in patients with moderate or mild disease (Supplementary Table 2 and Fig. 6b). Indeed, blood glucose levels in patients with COVID-19 were higher than those in the reference population and were particularly higher in patients with severe disease (Fig. 6c,d). Notably, the plasma GP73 levels in patients with COVID-19 were positively correlated with blood glucose levels (Fig. 6e). It should be noted that the levels of GP73 and glucose levels remained elevated after recovery before discharge (Fig. 6f,g).
a–d, Plasma GP73 levels (a) or glucose levels (c) in healthy controls and patients with COVID-19. P < 0.0001 for COVID-19 versus healthy by two-tailed Student’s t-tests (a,c). Plasma GP73 levels (b) or blood glucose levels (d) in healthy controls and patients with COVID-19 with different disease severities. P = 0.0198 for moderate versus healthy (b) and P < 0.0001 for severe versus healthy (b), P = 0.0193 for moderate versus healthy (d) and P < 0.0001 for severe versus healthy (d) by one-way ANOVA followed by Bonferroni’s post hoc test. e, Correlation analysis between plasma GP73 levels and glucose levels in patients with COVID-19. P < 0.0001 by Pearson correlation analysis. f,g, Glucose levels (f) or plasma GP73 levels (g) in patients with COVID-19 at admission, inpatient and after recovery. In the box-and-whisker plots, the box represents the upper and lower quartiles and the line within the box is the mean. The whiskers represent the maximum and minimum values. P < 0.0001 for admission versus healthy, inpatient versus healthy and recovery versus healthy (f,g) by one-way ANOVA followed by Bonferroni’s post hoc test. Data are mean ± s.e.m. *P < 0.05; ***P < 0.001.
GP73 blockade inhibits SARS-CoV-2-induced gluconeogenesis enhancement
To assess whether pathologically elevated GP73 contributes to SARS-CoV-2-induced glucose abnormalities, we monitored the effect of SARS-CoV-2 on gluconeogenic activity. As expected, the induction of glucogenic gene expression and hepatocyte glucose production was significantly increased by SARS-CoV-2 infection (Fig. 7a,b). In contrast, the upregulated glucogenic gene expression and hepatocyte glucose production were all markedly reduced by anti-GP73 antibody treatment (Fig. 7a,b), similar to the effect of GP73 depletion (Fig. 7c,d). Meanwhile, PKA activation by SARS-CoV-2 infection was also significantly blocked by a GP73-specific antibody (Fig. 6e). To further investigate whether the changes in serum GP73 were closely associated with gluconeogenesis levels, we obtained serum from patients with COVID-19 with GP73 levels higher than 60 ng ml−1 and age-matched healthy individuals with GP73 levels lower than 20 ng ml−1 and added the samples to culture medium. Glucogenic gene expression and PKA enzymatic activity were more strongly induced in cells exposed to serum from patients with COVID-19 than in cells exposed to serum from healthy controls and these effects were significantly blocked by anti-GP73 antibody (Fig. 7f,g).
a,b, Glucogenic gene expression (a) or glucose levels (b) in Huh-7 cells infected with SARS-CoV-2 (MOI 0.1) for 24 h with or without GP73 antibody (1 µg ml−1). P = 0.0004 for infected versus mock (Pc) and P = 0.0001 for anti-GP73 versus isotype (Pc, infected), P = 0.0002 for infected versus mock (Fbp1) and P < 0.0001 for anti-GP73 versus isotype (Fbp1, infected), P < 0.0001 for infected versus mock (G6pc) and P < 0.0001 for anti-GP73 versus isotype (G6pc, infected), P < 0.0001 for infected versus mock (Pck1) and P < 0.0001 for anti-GP73 versus isotype (Pck1, infected) by one-way ANOVA followed by Bonferroni’s post hoc test (a), P < 0.0001 for infected versus mock and P = 0.0013 for anti-GP73 versus isotype by one-way ANOVA followed by Bonferroni’s post hoc test (b). c,d, Glucose levels (c) or hepatic glucogenic gene expression (d) in WT and GP73 KO-1 Huh-7 cells infected with SARS-CoV-2 (MOI 0.1) for 24 h in glucose-free medium. P = 0.0002 for infected versus mock (WT) and P = 0.0014 for GP73 KO versus WT (infected) by one-way ANOVA followed by Bonferroni’s post hoc test (c). P < 0.0001 for infected versus mock (G6pc, WT) and P = 0.0001 for GP73 KO versus WT (G6pc, infected), P < 0.0001 for infected versus mock (Pck1, WT) and P = 0.0002 for GP73 KO versus WT (Pck1, infected) by one-way ANOVA followed by Bonferroni’s post hoc test (d). e,f, Immunoblotting analysis of PKA-C-α phosphorylation and substrate levels in Huh-7 cells treated with SARS-CoV-2 or the indicated serum. g, Glucogenic gene expression in Huh-7 cells cultured with the indicated serum with or without GP73 antibody. P < 0.0001 for COVID-19 versus healthy (Pc, Fbp1, G6pc and Pck1) and P < 0.0001 for anti-GP73 versus isotype (Pc, Fbp1, G6pc and Pck1; COVID-19) by one-way ANOVA followed by Bonferroni’s post hoc test. h,i, Fasting glucose levels and hepatic G6pc mRNA expression in mice inoculated with 1.6 × 104 p.f.u. of MASCp6 and killed at day 2 after inoculation (n = 6). P = 0.0213 for anti-GP73 versus isotype (mock), P = 0.0030 for infected versus mock and P = 0.0011 for anti-GP73 versus isotype (infected) by one-way ANOVA followed by Bonferroni’s post hoc test (h). P = 0.0152 for anti-GP73 versus isotype (mock), P = 0.0012 for infected versus mock and P < 0.0001 for anti-GP73 versus isotype (infected) by one-way ANOVA followed by Bonferroni’s post hoc test (i). Animal and cell-based studies were performed independently at least three biological replicates with comparable results. Data are mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001.
We next demonstrated the relevance of these in vitro findings in a MASCp6-infected BALB/c mouse model. Compared with mock-infected control mice, MASCp6-infected mice exhibited elevated fasting blood glucose levels and increased hepatic G6pc expression on day 2 after inoculation (Fig. 7h,i). In contrast, pretreatment of MASCp6-infected mice with GP73-specific antibody led to a significant reduction in fasting blood glucose levels to approximately the same level as that seen in mock-infected mice (Fig. 7h). The upregulated G6pc expression in livers from MASCp6-infected mice was also markedly reduced by anti-GP73 antibody pretreatment (Fig. 7i). Thus, GP73 blockade inhibits SARS-CoV-2-induced increases in gluconeogenesis in vitro and lowers elevated fasting blood glucose levels in SARS-CoV-2-infected mice.
Discussion
The precise regulation of gluconeogenesis required for physiological adaptation to fasting and starvation and occurs at multiple levels6,25. In response to stimulation by external factors, circulating blood levels of insulin, glucagon and glucocorticoids change, leading to subsequent changes in glucogenic pathways and glucose production26. Here, we provide evidence linking circulating GP73 to hepatic gluconeogenesis. Consistent with the fact that hepatic glucose release is necessary during fasting, circulating concentrations of GP73 rise during fasting. Fasted mice in which GP73 is blockade fail to maintain normal blood glucose levels during the fasting period. Experimentally modulating the circulating GP73 concentration via direct recombinant protein injection or immunologic sequestration results in corresponding changes in fasting plasma glucose. Mechanistically, secreted GP73 primarily targets the liver to promote gluconeogenesis via activation of PKA signaling in endocrine and autocrine manners. Notably, plasma GP73 levels are pathologically elevated in patients with COVID-19 and GP73 is essential for enhanced gluconeogenesis in response to SARS-CoV-2 infection. We thus proposed that the glucogenic property of GP73 might contribute to the hyperglycemia that occurs during SARS-COV-2 infection and in patients with COVID-19.
We assessed the direct actions of GP73 in the regulation of glucose production in primary hepatocytes in the absence of other counterregulatory hormones. GP73 was sufficient to cause cAMP accumulation, stimulate the enzymatic activity of PKA, increase the phosphorylation of CREB and enhance the transcriptional expression of Pcx, Pck1 and G6pc. These results suggest that GP73 stimulates gluconeogenesis via the activation of PKA signaling through transcriptional regulation. In support of this hypothesis, we observed drastic remodeling of the PKA hub following GP73 challenge using phosphoproteomics. Likewise, phosphosites that were upregulated by glucagon were also significantly enriched with substrate motifs for PKA, indicating that glucagon and GP73 have overlapping functions at the signaling level. Among the potentially functional phosphosites regulated by GP73 and GCG, Itpr1 plays a crucial role in glucagon-stimulated hepatic gluconeogenesis, driving a metabolic program by stimulating intrahepatic lipolysis and increasing the mitochondrial fat oxidation process27. Therefore, GP73 may acutely stimulate gluconeogenesis by activating intrahepatic or WAT lipolysis in a transcription-independent alternative mechanism. Serving as a stimulator of HGP, a clamp study in vivo and insulin-induced AKT phosphorylation in vitro demonstrated a role of GP73 in regulating peripheral insulin sensitivity. The unaltered ITT indicates that GP73 might regulate insulin secretion in a context-dependent manner. Nevertheless, illustrating the potential role of GCGR as a receptor of GP73 to modulate HGP and identifying other potential cell-surface receptors through which GP73 exerts its effects may provide more mechanistic information.
Epidemiological and experimental data indicate that SARS-CoV-2 infection is associated with new-onset diabetes28,29. The mechanisms underlying this phenomenon are likely complex and may include the promotion of inflammation, structural lung damage and systemic effects30. The present study reported that SARS-CoV-2 infection was associated with insulin resistance via pathways involved in endogenous glucose production31. We demonstrated that enhanced gluconeogenic metabolism following SARS-CoV-2 infection was primarily dependent on GP73. In patients with COVID-19, as opposed to significantly elevated GP73 levels in patients with severe disease, serum GP73 levels in patients with mild disease were comparable to those observed in healthy controls, suggesting the presence of a threshold of disease severity or chronicity required for GP73 upregulation. Although the SARS-CoV-2 N and S proteins stimulated GP73 secretion, our data raise the question of whether GP73 expression is limited to SARS-CoV-2 replication. In fact, the levels of GP73 remained elevated after recovery. It has been observed that hyperglycemia persists for 3 years after recovery from SARS and can be detected for at least 2 months in patients recovered from COVID-19 (refs. 13,32). A previous study showed that GP73 production is activated and correlated with interferon-β activation during RNA viral infection through MAVS33. Potential mechanisms for the regulation of GP73 expression demonstrated the involvement of cytokines, liver damage and mTOR signaling34,35,36. Therefore, GP73 expression may be controlled in SARS-CoV-2-dependent and SARS-CoV-2-independent manners. It should be noted that HFD and HBV/HCV infection also induce circulating GP73 levels37,38. As a Golgi-resident protein exhibiting glucoregulatory actions, the potential contribution of GP73 to glucose abnormalities in the context of infection with other viruses, or in metabolic diseases, including diabetes, warrants further investigation. Apart from the role of GP73 in promoting cell proliferation, tumor development and metastasis, GP73 also represses the host innate immune response to promote RNA virus replication33,39. Therefore, long-term follow-up of infected patients is warranted. Due to the urgent circumstance of the COVID-19 pandemic, we were unable to retrieve more clinical data including information on body mass index, steatohepatitis and fibrosis status from the current patient cohort. Although all patients were HBV, HCV and HIV negative, the possibility that NASH might have influenced GP73 release cannot be fully excluded.
In summary, we provide evidence that SARS-CoV-2 infection induces GP73 production and secretion, which results in an exaggerated gluconeogenic response. Sustained elevated GP73 may directly predispose the host to abnormal glucose metabolism (Extended Data Fig. 10). Our findings suggest that neutralizing plasma GP73 might be therapeutic option for treating patients with SARS-CoV-2 infection that warrants further investigation.
Methods
Reagents
Sodium l-lactate (71718), sodium pyruvate (792500) and aprotinin (A6106) were purchased from Sigma-Aldrich. Insulin (2018283062) was purchased from Novo Nordisk. Glucagon (HY-P0082) and FSK (HY-15371) were purchased from MedChemExpress. Glucose (20171108) was purchased from Sinopharm Chemical Reagent. A blood glucose meter (06656919032) and test strips (1072332990) were purchased from Roche. Sulfo-cyanine7 NHS ester (Cy7, GY1058) was purchased from Goyoo Biotechnology. Dulbecco’s modified Eagle’s medium (DMEM; high-glucose, D5796), protease inhibitor cocktail I (20-201) and a dipeptidyl peptidase-4 (DPP4) inhibitor (DPP4-010) were purchased from Millipore. Low-glucose DMEM (31600-500), glucose-free DMEM (90113-500) and l-alanine (A8210) were purchased from Solarbio. Fetal bovine serum (FBS; A3160901) was purchased from Gibco. Lipofectamine 2000 (11668-027) was purchased from Invitrogen. Chow (HD1001) and HFD (HD001) were purchased from BiotechHD. H89 (S1643) was purchased from Beyotime, LY2409021 (HY-19904) was purchased from MedChemExpress and cAMPS-Rp triethylammonium salt (151837-09-1) was purchased from Tocris Bioscience. Collagenase IV (2091) was purchased from BioFroxx. PerfectStart Green qPCR SuperMix (AQ601) and TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (AT311) were purchased from TransGen Biotech. NucleoZOL (740404) was purchased from MACHEREY-NAGEL. A mouse insulin ELISA kit (PI602) and BCA protein concentration determination kit (P0012) were purchased from Beyotime. A cAMP assay kit (ab133051) and PKA Kinase Activity kit (ab139435) were purchased from Abcam. An Amplex Red Glucose/Glucose Oxidase Assay kit (A22189) was purchased from Invitrogen.
Antibodies
Anti-α-tubulin (T6074, 1:5,000 dilution) and anti-Flag (A8592, 1:5,000 dilution) antibodies were purchased from Sigma-Aldrich. An anti-GP73 antibody (F-12, sc-393372, 1:200 dilution) was purchased from Santa Cruz. An anti-His antibody (KM8001, 1:1,000 dilution) was purchased from Taihua Lekang Biotechnology. Anti-phospho-PKA-C-α (Thr197, 5661, 1:1,000 dilution), anti-phospho-PKA substrate (RRXpS/T, 9624, 1:1,000 dilution) and anti-PKA-C-α (5842, 1:1,000 dilution) antibodies were purchased from Cell Signaling Technology. Anti-rabbit HRP-IgG (ZB-2301, 1:5,000 dilution) and anti-mouse HRP-IgG (ZB-2305, 1:5,000 dilution) secondary antibodies were purchased from ZSGB-BIO. Anti-spike (GTX632604, 1:500 dilution) antibody was purchased from GeneTex. An anti-GP73 monoclonal antibody for the blocking experiment was custom made. Isotype-matched IgG (A7028) was purchased from Beyotime.
Plasmids and cell culture
Mammalian expression vectors encoding Flag-tagged human, mouse and rat GP73 were constructed by inserting the corresponding PCR-amplified fragments into pcDNA3 (Invitrogen). The 23 genes of SARS-CoV-2 (Wuhan-Hu-1, GenBank, NC_045512.2) were optimized and cloned into the pXJ2 expression vector with a 3× Flag tag at the C terminus as previously described40. All constructs were verified by DNA sequencing. An expression plasmid for human Flag-ACE2 (H3673) was obtained from Vigene Biosciences. The HepG2 (CRL-10741), Caco-2 (HTB-37), A549 (CCL-185), Vero E6 (CRL-1586), L6 (CRL-1658) and 293T (CRL-3216) cell lines were obtained from the American Type Culture Collection. The Huh-7 (0403) cell line was obtained from the Japanese Collection of Research Bioresources. All cell lines were tested for mycoplasma contamination and were incubated in DMEM at 37 °C in a humidified atmosphere with 5% CO2. Lipofectamine 2000 was used for transfection following the manufacturer’s protocol.
To knock out human GP73 in Huh-7 cells, two small guide RNAs (sgRNAs) targeting GP73 were designed and inserted into the LentiCrispr v2 vector to construct transfer plasmids. 293T cells were transfected with pMD2. G, psPAX2 and the corresponding transfer plasmid to produce lentivirus. A total of 108 Huh-7 cells were infected with lentivirus at an MOI of 2.0 and selected with 4 μg ml−1 puromycin for 2 weeks to ensure proper selection.
The following sgRNA sequences were used:
sgRNA-1: 5ʹ-CACCGCACACACAGAGGTGCCACAA-3ʹ
sgRNA-2: 5ʹ-CACCGACCAGTTAAAGACCCTGCAG-3ʹ
control- 5ʹ-CACCGCGCTTCCGCGGCCCGTTCAA-3ʹ.
PMHs were isolated and purified using a modified two-step collagenase perfusion method. Cells were resuspended in low-glucose DMEM containing 5% FBS and seeded on 15-cm dishes at 80% confluence. Five hours later, the cells were washed and cultured in serum-free medium overnight. For gluconeogenesis-related assays, the medium was replaced with glucose-free and phenol-free DMEM the next day in the presence of 10 mM pyruvate sodium and 10 mM sodium lactate and the cells were treated with the indicated concentrations of rmGP73 or rrGP73, 200 mM cAMPS-Rp or 2 μM glucagon.
Sample acquisition from patients with COVID-19
The Ethics Committee of Huoshenshan Hospital approved the study (HSSLL036). Given the urgency of the COVID-19 pandemic, the need for informed consent forms was waived by the ethics boards of the hospitals. Basic information, serum biochemical test results and blood samples for GP73 and glucose level analysis were collected from 76 patients with COVID-19 at Huoshenshan Hospital from 11 January to 11 March 2020 at admission before any intervention. Diagnosis was based on chest computed tomography (CT) manifestations and/or PCR with reverse transcription (RT–PCR) according to the criteria of the New Coronavirus Pneumonia Prevention and Control Program (5th edition) published by the National Health Commission of China. According to these criteria, patients with COVID-19 were classified into mild, moderate and severe COVID-19 subgroups. The majority of patients received combined treatments, including antiviral drugs (lopinavir/ritonavir, interferon-α and arbidol), herbs, intravenous immunoglobulin and antibiotics. Data were excluded if the patient was younger than 18 years or older than 75 years, had incomplete medical records, acute lethal organ injury (for example, acute myocardial infarction, acute coronary syndrome, acute pulmonary embolism or acute stroke) or decompensated/end-stage chronic organ dysfunction (for example, decompensated cirrhosis, decompensated chronic renal insufficiency or severe congestive heart failure), was pregnant, was HBV-, HCV- or HIV-positive, used drugs with known effects on glucose metabolism (glucocorticoid or tocilizumab) or had malignancy (Supplementary Table 1). Twenty-eight patients had mild COVID-19, 36 patients had moderate COVID-19 and 12 patients had severe COVID-19 (Supplementary Table 2). Among them, four patients with moderate COVID-19 and nine patients with severe COVID-19 were enrolled and blood samples were collected at admission before any intervention, during hospitalization and after recovery before discharge. The discharge criteria included normal temperature, resolved respiratory symptoms, improved CT images and two consecutively negative RT–PCR results for SARS-CoV-2 in respiratory tract specimens within an interval of at least 24 h.
Recombinant GP73 protein purification
Human, mouse and rat GP73 complementary DNAs, each with a six-amino-acid His tag on the N terminus, were cloned into the pCDNA3.1 vector. Ni-NTA His-Bind column-bound His-GP73 protein from 293T cells transfected with the above plasmids was further purified using size-exclusion columns and polymyxin B-based endotoxin-depletion columns after extensive washing. The final His-GP73 proteins used in all recombinant protein experiments were >90% pure (endotoxin ≤2 EU ml−1) and stored at −80 °C.
Animals, intervention and monitoring
Male C57BL/6N WT mice (8–10 weeks old) were purchased from SPF Biotechnology. Male GP73 KO mice (T20200316-18[D25], 8–10 weeks old) were generated from C57BL/6N WT mice by Southern Model Biotechnology. All mice were group-housed conventionally on a 12-h light/dark cycle for 3 d before any experiments, the environmental conditions were maintained thermostatically between 18 °C −23 °C with 40–60% humidity. All animal experiments were performed at the AMMS Animal Center and were approved by the Institutional Animal Care and Use Committee. For single injection, mice were injected i.v. with 0.1 mg kg−1 rmGP73 and plasma was collected at the indicated times via tail bleeding for insulin and glucose level measurements. The ITT, GTT, PTT and ATT were performed using standard procedures. A 0.75 U kg−1 insulin bolus was used for the ITT, a 1.5 g kg−1 glucose bolus was used for the GTT, a 1.5 g kg−1 pyruvate bolus was used for the PTT and a 0.6 g kg−1 alanine bolus was used for the ATT. For immunological sequestration experiments, mice were injected i.v. with 15 mg kg−1 custom-made anti-GP73 mouse monoclonal antibody or an equivalent dose of IgG (30 mg kg−1).
Glucose measurement
All blood samples were collected from the tail and glucose levels were measured using the glucose oxidase method and an automated blood glucose reader (ACCU-CHEK, Roche). For the measurement of fasting blood glucose levels, normal mice were fasted for 6 or 12 h as indicated. Random blood glucose levels were measured at 9:00 a.m. If the glucose level was >630 mg dl−1 (upper detection limit of the glucometer), a value of 630 mg dl−1 was recorded. Blood glucose levels were determined.
Assays of plasma hormone levels
Blood samples for hormone detection were collected from the tail or orbital vein. A DPP4 inhibitor (1:100 dilution), aprotinin (1:100 dilution) and protease inhibitor cocktail I (50,000 KIU ml−1, 1:100 dilution) were added to each blood sample. Plasma insulin levels were measured using ELISA.
Hyperinsulinemic-euglycemic clamp
The assays were performed as previously described41. Mice were anesthetized with an intraperitoneal injection of 50 mg kg−1 pentobarbital sodium salt (Sigma, P3761). Hairs at the incision site were removed by an electric hair clipper. An incision was made in the skin and the right jugular vein was identified. A catheter (Instech Laboratories, C10PU-MCA1301) filled with heparinized saline (200 U heparin per ml saline) was inserted into the vein toward the side of the chest. A suture was placed at each end of the vessel. The catheter was tunneled under the skin from the right jugular to the interscapular incision on the back. The catheter was plugged with a mouse button harness (Instech Laboratories, VABM2B/22R22) and settled on the back. All skin wounds were sutured and the mouse recovered for 3–4 d. Mice that lost <5% of their precannulation weight after recovery were used for clamp studies.
The mice were fasted overnight, weighed to calculate the insulin dose and placed in a transparent plastic terrarium (Instech Laboratories, STANK/W). The setup and timeline for the experiment are shown in Extended Data Fig. 6a. An equilibration syringe pump was connected to the catheter and GFP or GP73 was administered at t = −150 min. At t = −90 min, [6,6-2H] glucose (600 μg kg−1) was administered, followed by continuous infusion of [6,6-2H] glucose (30 μg kg−1 min−1). Isotopic enrichment was achieved at approximately 60 min after the onset of isotope infusion and blood samples for isotope measurements were collected at t = −10 and 0 min. Following this basal infusion period, insulin was constantly infused at a rate of 6 mU kg−1 min−1 until termination of the study. [6,6-2H]glucose was infused together with glucose at various rates until the blood glucose concentration reached a constant level of approximately 100 ± 10 mg dl−1. Blood samples were taken at t = 110 and 120 min.
Mass spectrometry
Mass spectrometry was performed at the Protein Preparation and Characterization Platform of the Tsinghua University Technology Center for Protein Research as previously described41. Plasma samples (15 μl) were deproteinized by gently mixing them with 60 μl cold methanol (prechilled at −80 °C) and incubating for 1–2 h at −80 °C. The samples were then centrifuged at 14,000g at 4 °C for 10 min. The supernatant was transferred to a new tube and lyophilized to produce a pellet. High-resolution mass spectrometry (Q ExactiveHFX, Thermo) coupled with Vanquish UHPLC was used for the analysis. Negative ion mode with a mass resolution of 120,000 was performed for glucose detection. The flow rates of sheath gas and aux gas were set as 35 and 10, respectively. Spray voltage was 2.8 kV. For LC separation, an amide column (2.1 mm × 100 mm, Waters) was used for analysis of glucose. Column temperature was 40 °C. Mobile phases contained 80% acetonitrile in A and 30% acetonitrile in B with 5 mM ammonium acetate as a modifier. The flow rate was 250 μl min−1 and the gradient was as follows: 0–3 min: 10% B; 3–4 min: 10–60% B; 4–6 min: 60% B; 6.1–8 min: 10% B. A total of 2 μl of sample was injected for analysis. Accurate masses of 179.0561 and 181.0686 with a mass tolerance of 3 ppm were used for the extraction of glucose and [6,6-2H]glucose in negative mode. For the calibration curve, concentrations of 5, 10, 25, 50, 100, 250, 500, 1,000, 2,500, 5,000, 10,000, 25,000 ng ml−1 for [6,6-2H]glucose and 1, 2, 5, 10, 20, 50, 100, 200, 500, 1,000, 2,500, 5,000 μg ml−1 for glucose were prepared in 80% methanol.
Calculation
All calculations are based on Steele’s equations for steady-state conditions41,42. Under steady-state conditions, the rate of glucose appearance (Ra) is equal to the rate of disappearance (Rd)43. The GDR is equal to the constant isotope infusion rate (F) divided by isotope enrichment (atom percent excess, APE) minus F. There is no glucose infusion during the basal infusion period, so GDR is equal to the rate of HGP. During infusion of unlabeled glucose mixed with [6,6-2H] glucose, GDR is equal to the sum of the rate of HGP and the glucose infusion rate (GIR). The HGP is equal to GDR minus GIR. The calculation was performed as indicated in Extended Data Fig. 6b.
Immunofluorescence staining
Tissues were fixed with 10% (v/v) neutral-buffered formalin at 4 °C overnight and embedded in paraffin and 5-μm-thick sections were prepared. For immunofluorescence, the sections were heated in an autoclave in citrate buffer (12 mmol l−1, pH 6.0), preincubated in permeabilization/blocking buffer (0.1 mmol l−1 PBS, pH 7.3, 0.5% Triton) and blocked for 30 min with 10% (v/v) goat serum (Zhongshan Biotechnology). The sections were subsequently incubated with primary antibodies at 4 °C overnight and secondary antibodies for 1 h at room temperature, washed and stained with 4,6-diamidino-2-phenylindole (1 μg ml−1). Images were captured under a confocal fluorescence microscope (Zeiss LSM710, Carl Zeiss Microscopy) or an automatic digital slide scanner (Pannoramic MIDI, 3D HISTECH). Nikon NIS-Elements AR 4.00.12 and CaseViewer 2.4 software were used to analyze the image data.
Phosphoproteomics
PMHs were suspended in low-glucose DMEM containing 5% FBS and seeded in 15-cm dishes at 80% confluence. Cells were washed and cultured in serum-free medium overnight 5 h later. The medium was replaced with glucose-free and phenol-free DMEM supplemented with 10 mM pyruvate sodium and 10 mM sodium lactate the next day and the cells were incubated for 1 h with PBS, rmGP73 (64 nM) or glucagon (2 μM). For cell lysate collection, the cells were washed twice with cold PBS and scraped with cold RIPA lysis buffer supplemented with protease and phosphatase inhibitors. Phosphoproteomics was performed by Oebiotech Company. Briefly, samples were subjected to enzyme digestion and iTRAQ labeling and the phosphopeptides were enriched and analyzed using LC–MS/MS. The raw data of this study have been deposited in the IProX database under accession no. PXD025381. For kinase enrichment, the Literature Based Kinase–Substrate Library with Phosphosites on the Kinase Enrichment Analysis 2 (KEA2) website was searched (https://www.maayanlab.net/KEA2/). The network was represented using Cytoscape v.3.6.2 (https://cytoscape.org/). Protein–protein interactions were retrieved from STRING App (v.1.51) (https://string-db.org/). Only interactions with high confidence (interaction score >0.7) from databases and experiences were kept. For KEGG and Gene Ontology enrichment analysis, DAVID Bioinformatics Resources 6.8 (https://david.ncifcrf.gov/home.jsp) was used. For specific kinase–substrate motif analysis, MoMo Modification Motifs 5.3.3 (https://meme-suite.org/tools/momo) was used.
In vivo imaging system
GP73 was labeled with Cy7 via the addition of the dye according to the manufacturer’s instructions at pH 8.0 and incubation of the mixture for 4 h on ice. Labeled GP73 was returned to pH 7.0 and the free dye was removed via overnight dialysis in PBS. The labeled GP73 was added to a Sephadex G50 size-exclusion column equilibrated with PBS. Fractions of 500 µl were collected, the protein concentration was analyzed using a standard BCA protein assay kit and fluorescence corresponding to excitation/emission of 745/800 nm was assessed.
After i.v. injection with free Cy7 and GP73-Cy7, mice were scanned using an IVIS (PerkinElmer) at the indicated time points to assess fluorescence. After whole-body imaging, the mice were sacrificed and the major organs were imaged to assess fluorescence under the same settings as the in vivo imaging. The data were analyzed and exported using built-in Living Image Software (v.4.5.5, PerkinElmer).
Quantitative real-time PCR
Total mRNA was extracted from cells or various mouse tissues using NucleoZOL. cDNA was prepared from total mRNA using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix and the relative levels of individual mRNAs were calculated after normalization to the GAPDH mRNA level in the corresponding sample as previously described. Primer sequences are presented in Supplementary Table 3.
Sandwich ELISA and western blot analysis
For GP73 sandwich ELISA, two custom-made rat monoclonal anti-GP73 antibodies were used as the capture antibody and the detection antibody. Briefly, the plate was coated with an unlabeled capture antibody and serially diluted standards and samples were added to the plate. After three washes, HRP-linked detection antibody was added to generate a colorimetric signal at 450 nm. Increasing amounts of recombinant His-tagged GP73 were used to generate a standard curve.
For immunoblotting, cells were lysed in NP40 cell lysis buffer with fresh protease inhibitors. Whole-cell lysates were separated using SDS–PAGE after centrifugation and transferred to polyvinylidene difluoride membranes for immunoblot analyses using the indicated primary antibodies.
Infection with SARS-CoV-2
All procedures involving cells and animals were conducted in the Biosafety Level 3 laboratory (BSL-3) and approved by the Animal Experiment Committee of Laboratory Animal Center, Beijing Institute of Microbiology and Epidemiology (approval number: IACUC-DWZX2020-002). Animal studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals.
The SARS-CoV-2 strain (BetaCoV/Beijing/IME-BJ01/2020) used for cell infection in the present study was isolated from the lung lavage fluid of an infected patient and preserved at the State Key Laboratory of Pathogen and Biosecurity at Beijing Institute of Microbiology and Epidemiology. The infectious virus titer was determined as p.f.u. in Vero E6 cells and was used to calculate the MOI. Cells were infected with SARS-CoV-2 at the indicated MOIs for the indicated times in glucose-free medium for the production assay. The results were normalized to the protein content. Cells were infected with SARS-CoV-2 at the indicated MOIs for the indicated time and the supernatant was collected for the GP73 assay and viral load assay. Viral RNA was extracted from supernatants using the QIAamp Viral RNA Mini kit (52906, QIAGEN) according to the manufacturer’s instructions. Viral RNA was analyzed using qRT–PCR and a One-Step PrimeScript RT–PCR kit (RR064B, TaKaRa) using SARS-CoV-2-specific primers in an Applied Biosystems 7500 Real-time PCR System. The following sequences of the SARS-CoV-2 probes were used:
SARS-CoV-2 open reading frame 1b (ORF1b):
Forward: 5ʹ- CCCTGTGGGTTTTACACTTAA-3ʹ
Reverse: 5ʹ- ACGATTGTGCATCAGCTGA-3ʹ.
Probe: 5ʹ-FAM- CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ 1-3ʹ.
SARS-CoV-2 nucleocapsid (N):
Forward: 5ʹ- GGGGAACTTCTCCTGCTAGAAT-3ʹ
Reverse: 5ʹ- CAGACATTTTGCTCTCAAGCTG-3ʹ.
Probe: 5ʹ-FAM- TTGCTGCTGCTTGACAGATT-BHQ 1-3ʹ.
The number of copies per μl was determined using a synthetic RNA fragment to amplify the target region.
The mouse-adapted SARS-CoV-2 strain MASCp6 used for mouse infection in the present study was from the Beijing Institute of Microbiology and Epidemiology and was stored at −80 °C. Nine-month-old male BALB/c mice were inoculated intranasally with MASCp6 (6 × 103 p.f.u. per mouse). For the measurement of fasting blood glucose levels, infected mice were fasted for 6 h on day 2 after infection, blood samples were collected from the tail and glucose levels were measured as uninfected mice. For mice samples collection, the sera were collected and mice were killed. Lung and liver tissues were subjected to viral RNA load analysis and immunohistochemistry analysis for GP73. Copies per ml were determined using a synthetic RNA fragment to amplify the target region.
Pseudovirus production
All pseudoparticles were generated in 293T cells transfected with the HIV backbone vector pNL4-3.Luc. R-E together with the plasmids encoding SARS-2-S or VSV-G as previously described.
Statistical analysis
Microsoft Office Standard 2010 was used for data calculation and GraphPad Prism 8.0 was used for statistical calculations and data plotting. Data are presented as mean ± s.e.m. Differences between two independent samples were evaluated using two-tailed Student’s t-tests or the Mann–Whitney U-test, as appropriate. Differences between multiple samples were analyzed using one-way or two-way ANOVA followed by Bonferroni’s post hoc test, as appropriate. Correlations were analyzed using Spearman’s nonparametric test. All tests were two-tailed unless otherwise indicated. We considered a P value ≤ 0.05 to be statistically significant. Significance values were set as follows: NS, not significant, P > 0.05; *P < 0.05; **P < 0.01; and ***P < 0.001.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The numeric source data are provided in source data files and all of the other data that support the findings of this study are available from the corresponding author upon reasonable request. The phosphoproteomics data have been deposited in iProX database and are accessible through accession number PXD025381. All online datasets used in this study including KEGG (https://www.genome.jp/kegg/), DAVID (https://david.ncifcrf.gov/home.jsp), Cytoscape (https://cytoscape.org/) MoMo (https://meme-suite.org/tools/momo), STRING (https://string-db.org/) and KEA2 (https://www.maayanlab.net/KEA2/). Source data are provided with this paper.
References
Roder, P. V., Wu, B., Liu, Y. & Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med 48, e219 (2016).
Sharabi, K., Tavares, C. D., Rines, A. K. & Puigserver, P. Molecular pathophysiology of hepatic glucose production. Mol. Asp. Med. 46, 21–33 (2015).
Chadt, A. & Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflug. Arch. 472, 1273–1298 (2020).
Kowalski, G. M. & Bruce, C. R. The regulation of glucose metabolism: implications and considerations for the assessment of glucose homeostasis in rodents. Am. J. Physiol. Endocrinol. Metab. 307, E859–E871 (2014).
Chung, S. T., Chacko, S. K., Sunehag, A. L. & Haymond, M. W. Measurements of gluconeogenesis and glycogenolysis: a methodological review. Diabetes 64, 3996–4010 (2015).
Zhang, X., Yang, S., Chen, J. & Su, Z. Unraveling the regulation of hepatic gluconeogenesis. Front. Endocrinol. 9, 802 (2018).
Fleig, W. E., Noether-Fleig, G., Roeben, H. & Ditschuneit, H. Hormonal regulation of key gluconeogenic enzymes and glucose release in cultured hepatocytes: effects of dexamethasone and gastrointestinal hormones on glucagon action. Arch. Biochem. Biophys. 229, 368–378 (1984).
Deng, L. et al. Hepatitis C virus infection promotes hepatic gluconeogenesis through an NS5A-mediated, FoxO1-dependent pathway. J. Virol. 85, 8556–8568 (2011).
Petersen, M. C., Vatner, D. F. & Shulman, G. I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 13, 572–587 (2017).
Cristelo, C., Azevedo, C., Marques, J. M., Nunes, R. & Sarmento, B. SARS-CoV-2 and diabetes: new challenges for the disease. Diabetes Res Clin. Pr. 164, 108228 (2020).
Lim, S., Bae, J. H., Kwon, H. S. & Nauck, M. A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat. Rev. Endocrinol. 17, 11–30 (2021).
Sathish, T. & Chandrika Anton, M. Newly diagnosed diabetes in patients with mild to moderate COVID-19. Diabetes Metab. Syndr. 15, 569–571 (2021).
Montefusco, L. et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 3, 774–785 (2021).
Ayres, J. S. A metabolic handbook for the COVID-19 pandemic. Nat. Metab. 2, 572–585 (2020).
Alves, A. M., Yvamoto, E. Y., Marzinotto, M. A. N., Teixeira, A. C. S. & Carrilho, F. J. SARS-CoV-2 leading to acute pancreatitis: an unusual presentation. Braz. J. Infect. Dis. 24, 561–564 (2020).
Liu, F. et al. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin. Gastroenterol. Hepatol. 18, 2128–2130 (2020).
Muller, J. A. et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 3, 149–165 (2021).
Kladney, R. D. et al. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene 249, 53–65 (2000).
Hu, L., Li, L., Xie, H., Gu, Y. & Peng, T. The Golgi localization of GOLPH2 (GP73/GOLM1) is determined by the transmembrane and cytoplamic sequences. PLoS ONE 6, e28207 (2011).
Puri, S., Bachert, C., Fimmel, C. J. & Linstedt, A. D. Cycling of early Golgi proteins via the cell surface and endosomes upon lumenal pH disruption. Traffic 3, 641–653 (2002).
Wei, C. et al. Tumor microenvironment regulation by the endoplasmic reticulum stress transmission mediator Golgi protein 73 in mice. Hepatology 70, 851–870 (2019).
Wang, L. et al. Serum Golgi protein 73 as a potential biomarker for hepatic necroinflammation in population with nonalcoholic steatohepatitis. Dis. Markers 2020, 6036904 (2020).
Nie, X. et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell 184, 775–791 (2021).
Gu, H. et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science 369, 1603–1607 (2020).
Romere, C. et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell 165, 566–579 (2016).
Yabaluri, N. & Bashyam, M. D. Hormonal regulation of gluconeogenic gene transcription in the liver. J. Biosci. 35, 473–484 (2010).
Perry, R. J. et al. Glucagon stimulates gluconeogenesis by INSP3R1-mediated hepatic lipolysis. Nature 579, 279–283 (2020).
Rubino, F. et al. New-onset diabetes in Covid-19. N. Engl. J. Med. 383, 789–790 (2020).
Hollstein, T. et al. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat. Metab. 2, 1021–1024 (2020).
Hussain, A., Bhowmik, B. & do Vale Moreira, N. C. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin. Pr. 162, 108142 (2020).
Govender, N., Khaliq, O. P., Moodley, J. & Naicker, T. Insulin resistance in COVID-19 and diabetes. Prim. Care Diabetes 15, 629–634 (2021).
Yang, J. K., Lin, S. S., Ji, X. J. & Guo, L. M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 47, 193–199 (2010).
Zhang, X. et al. GP73 represses host innate immune response to promote virus replication by facilitating MAVS and TRAF6 degradation. PLoS Pathog. 13, e1006321 (2017).
Chen, X. et al. mTORC1 up-regulates GP73 to promote proliferation and migration of hepatocellular carcinoma cells and growth of xenograft tumors in mice. Gastroenterology 149, 741–752 (2015).
Liang, H. et al. Interleukin-6 and oncostatin M are elevated in liver disease in conjunction with candidate hepatocellular carcinoma biomarker GP73. Cancer Biomark. 11, 161–171 (2012).
Liu, X. et al. Golgi protein 73(GP73), a useful serum marker in liver diseases. Clin. Chem. Lab. Med. 49, 1311–1316 (2011).
Kladney, R. D., Cui, X., Bulla, G. A., Brunt, E. M. & Fimmel, C. J. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology 35, 1431–1440 (2002).
Wolf, M. J. et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 26, 549–564 (2014).
Wang, Y. & Wan, Y. Y. Golgi protein 73, hepatocellular carcinoma and other types of cancers. Liver Res. 4, 161–167 (2020).
Zhang, J. et al. A systemic and molecular study of subcellular localization of SARS-CoV-2 proteins. Signal Transduct. Target Ther. 5, 269 (2020).
Zhang, Y., Xu, L., Liu, X. & Wang, Y. Evaluation of insulin sensitivity by hyperinsulinemic-euglycemic clamps using stable isotope-labeled glucose. Cell Discov. 4, 17 (2018).
Hother-Nielsen, O. & Beck-Nielsen, H. On the determination of basal glucose production rate in patients with type 2 (non-insulin-dependent) diabetes mellitus using primed-continuous 3-3H-glucose infusion. Diabetologia 33, 603–610 (1990).
Radziuk, J. & Lickley, H. L. The metabolic clearance of glucose: measurement and meaning. Diabetologia 28, 315–322 (1985).
Acknowledgements
We thank the National Key Research and Development Program of China (grant no. 2018YFA0900800) and National Natural Science Foundation of China (grant nos. 31872715 and 32070755) conducted by H. Zhong, the National Natural Science Foundation of China (grant nos. 81773205 and 82070595) conducted by C. Wei and the Postdoctoral Science Foundation of China (grant no. 2020M683743) conducted by X. Yang. A graphical abstract was created and exported with BioRender.com under a paid subscription.
Author information
Authors and Affiliations
Contributions
X.Y., C.Q., Z.S., F.W., C. Wei and H.Z. designed the experiments. C. Wei, H.Z., L.W., Q.G., G.C., E.M., X.Y. and H. Li collected and analyzed data. L.W., H.Y., H. Lin, J.G., Y.K., X.Y., L.H., Jing Liu, Y.X., Jingfei Li, C.L., F.Z., J.F., Q Yan, M.L. and Y.Z. carried out mice assays. Y.D., X.H., Q.C. and Q. Ye carried out authentic virus assays. L.W., E.M., H. Lin, C. Wang, Dongyu Li, H.W., L.X., Jialong Liu, X.Z., Y.Z., Dongrui Li, M.W., Y.M., K.T., Y.Y., Jianmin Li and H.H. carried out cell lines experiments. P.W. provided key experiment materials. X.Y., L.W., C. Wei and H.Z. analyzed the data and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Metabolism thanks John Nicholls, C. Ronald Kahn and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary handling editor: Christoph Schmitt.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 GP73 secretion is induced from multiple tissues upon fasting.
a-b, Intracellular GP73 (a) or supernatant GP73 levels (b) in HepG2 cells transfected with different concentrations of Flag-GP73. Cell-based studies were performed independently at least three times with comparable results. P = 0.0327 for 0.025 μg versus 0 μg, P < 0.0001 for 0.1 μg versus 0 μg, 0.4 μg versus 0 μg and 1.6 μg versus 0 μg by one-way ANOVA followed by Bonferroni’s post hoc test. Cell-based studies were performed independently at least three times with comparable results. The data were the means ± SEMs. *P < 0.05, ***P < 0.001.
Extended Data Fig. 2 Increase in circulating GP73 elevates fasting blood glucose.
a, Immunoblotting analysis (upper lane) and SDS-PAGE (lower lane) of purified His-tagged rmGP73 (56–401). b Immunoblotting analysis of purified His-tagged rmGP73 (56–401) and GP73 expression in 293 T cells transfected with the indicated plasmids. c, Mouse sandwich ELISA standard curve. d, Plasma GP73 levels in WT and GP73 KO mice (n = 4). P < 0.0001 for GP73 KO versus WT by two-tailed Student’s t-tests. e-f, Plasma GP73 levels in mice after rmGP73 injection at the indicated doses (e) for the indicated times (n = 3 or 4; f). P < 0.0001 for 0.2 mg/kg versus 0 mg/kg, 0.1 mg/kg versus 0 mg/kg and 0.05 mg/kg versus 0 mg/kg by one-way ANOVA followed by Bonferroni’s post hoc test (e). P < 0.0001 for 15 min versus 0 min, P < 0.0001 for 30 min versus 0 min, P = 0.0021 for 60 min versus 0 min by one-way ANOVA followed by Bonferroni’s post hoc test (f). Animal and cell-based studies were performed independently at least three times with comparable results. The data were the means ± SEMs. ***P < 0.01, ***P < 0.001.
Extended Data Fig. 3 Increase in circulating GP73 elevates fasting blood glucose.
a-d, Plasma glucagon (a), epinephrine (b), norepinephrine (c) and serum corticosterone (d) levels 15 min after rmGP73 or GFP injection (n = 3 or 4). ns, no significant for rmGP73 versus GFP by two-tailed Student’s t-tests (a-d). e, Glycogen levels in mice at 30 and 60 min after rmGP73 or GFP injection (n = 3 in each group). ns, no statistical significance for rmGP73 versus GFP (0 min, 30 min and 60 min) by two-tailed Student’s t-tests (a-d). f, Schematic representation of the metabolic process responsible for GP73-induced hyperglycemia. Animal studies were performed independently at least three times with comparable results. The data were presented as the means ± SEMs. ns, no statistical significance.
Extended Data Fig. 4 Circulating GP73 traffics to the liver and binds to the hepatocyte surface.
a, In vivo imaging of live anesthetized mice 30 min after i.v. injection of rmGP73-Cy7 or free Cy7 (n = 4). b, Tissue GP73 accumulation in Fig. 3a was measured as the photon intensity. P = 0.0129 for rmGP73-Cy7 versus free Cy7 (pancreas), P = 0.0019 for rmGP73-Cy7 versus free Cy7 (brain), P < 0.0001 for rmGP73-Cy7 versus free Cy7 (spleen, kidney and liver) by two-tailed Student’s t-tests. c, In vivo imaging of various organs from mice 1 h or 19 h after rmGP73-Cy7 or free Cy7 injection. Two representative images from four mice are shown. d, Liver GP73 accumulation in mice 1 h or 19 h after rmGP73-Cy7 or free Cy7 injection. P < 0.0001 for 1 h versus 19 h (rmGP73-Cy7) by one-way ANOVA followed by Bonferroni’s post hoc test. e, The level of biotin on the hepatocyte surface upon incubation of H22 cells with increasing concentrations of rhGP73-biotin or rmGP73-biotin with (nonspecific binding) or without (total binding) 100-fold excess recombinant GP73 in the medium was measured using a colorimetric assay. Specific binding (shown in red) was calculated as the difference between the two curves. Animal and cell-based studies were performed independently at least three times with comparable results. The data were the means ± SEMs. *P < 0.05; **P < 0.01; ***P < 0.001.
Extended Data Fig. 5 GP73 stimulates gluconeogenesis.
a, Glucose production in PMHs treated with 32 nM rmGP73 for the indicated times. P = 0.0159 for rmGP73 versus PBS (4 h), P = 0.0050 for rmGP73 versus PBS (8 h), P < 0.0001 for rmGP73 versus PBS (1 h and 2 h) by two-tailed Student’s t-tests. b, Glucose production in primary rat hepatocytes treated with the indicated concentrations of rrGP73 for 2 h. P < 0.0001 for 4 nM versus 0 nM, 8 nM versus 0 nM, 16 nM versus 0 nM, 32 nM versus 0 nM and 64 nM versus 0 nM by one-way ANOVA followed by Bonferroni’s post hoc test. c, Glucose production in PMHs treated with 32 nM rmGP73 with or without anti-GP73 antibody for 2 h. P = 0.0001 for rmGP73 versus PBS (isotype), P < 0.0001 for anti-GP73 versus isotype (rmGP73) by one-way ANOVA followed by Bonferroni’s post hoc test. d-e, Intracellular cAMP levels (d) or hepatocyte PKA activity (e) in PMHs treated with 32 nM rmGP73 for 2 h. P = 0.0031 for rmGP73 versus PBS (d) and P < 0.0001 for rmGP73 versus PBS (e) by two-tailed Student’s t-tests. Cell-based studies were performed independently at least three times with comparable results. The data were the means ± SEMs. *P < 0.05; **P < 0.01; ***P < 0.001.
Extended Data Fig. 6 GP73 stimulates gluconeogenesis.
a, Timeline of the procedure for performing the insulin clamp. The figure was created and exported with BioRender.com under a paid subscription. b, The equations used to calculate and evaluate insulin sensitivity. c-f, GP73 levels (c), blood glucose levels (d), GIR (e), and APE (f) in GP73 KO mice infused with GFP or recombinant GP73 during the clamp study. P < 0.0001 for rmGP73 versus GFP (basal and clamp) (c), P = 0.0029 for rmGP73 versus GFP (10 min), P = 0.0028 for rmGP73 versus GFP (20 min), P = 0.0068 for rmGP73 versus GFP (30 min), P = 0.0150 for rmGP73 versus GFP (40 min), P = 0.0489 for rmGP73 versus GFP (50 min), P = 0.0059 for rmGP73 versus GFP (60 min), P = 0.0258 for rmGP73 versus GFP (70 min), P = 0.0315 for rmGP73 versus GFP (80 min), P = 0.0137 for rmGP73 versus GFP (90 min), P = 0.0264 for rmGP73 versus GFP (100 min), P = 0.0049 for rmGP73 versus GFP (110 min), P = 0.0049 for rmGP73 versus GFP (120 min) by two-tailed Student’s t-tests (e), P = 0.0115 for rmGP73 versus GFP (basal) and P = 0.0048 for rmGP73 versus GFP (clamp) (f) by two-tailed Student’s t-tests. Hyperinsulinemic-euglycemic clamp studies were performed independently at least three times with comparable results. The data were the means ± SEMs. *P < 0.05; **P < 0.01; ***P < 0.001.
Extended Data Fig. 7 GP73 induces drastic remodeling of the PKA hub.
a, Distribution of matching kinases according to the phosphoproteomics data from the glucagon-treated sample (P < 0.05) using Kinase Enrichment Analysis 2 (KEA2) based on one-tailed Fisher’s exact test. b, GO enriched pathway analysis of significantly regulated phosphopeptides in PMHs treated with rmGP73 (P < 0.05) using DAVID Bioinformatics Resources 6.8. The bar plot shows significantly dysregulated pathways.
Extended Data Fig. 8 GP73 induces drastic remodeling of the PKA hub.
a-b, The kinase activity network was constructed according to the phosphoproteomics data from the GP73- (a) or glucagon- (b) treated samples using KEA2.
Extended Data Fig. 9 SARS-CoV-2 infection promotes GP73 secretion.
a-b, SARS-CoV-2 genome copy number per μL using specific primers for N (a) or ORF1b (b) in the supernatants of Huh-7 cells infected with SARS-CoV-2 for the indicated times and MOI. c, Cell viability of Huh-7 cells infected with SARS-CoV-2 at 0.1 MOI for the indicated times. d, SARS-CoV-2 viral RNAs in liver and lung tissues from mice inoculated with 1.6 × 104 PFU of MASCp6 and sacrificed at day 2 after inoculation (n = 6). e, Representative of three images of immunofluorescence staining for GP73 and SARS-CoV-2 S in paraffin-embedded lung tissue from three mice inoculated with 1.6 × 104 PFU of MASCp6 and sacrificed at day 3 after inoculation. f, Immunoblotting analysis of GP73 expression in Huh-7 cells transfected with plasmids expressing the indicated SARS-CoV-2 proteins. g, Immunoblotting analysis of SARS-CoV-2 N expression in Huh-7 cells transfected with different concentrations of Flag-N. h, Supernatant GP73 levels in the indicated human cell lines challenged with SARS-CoV-2pp or VSV-Gpp for 24 h. P < 0.0001 for SARS-2-Spp versus VSV-Gpp (HepG2/ACE2), P = 0.0035 for SARS-2-Spp versus VSV-Gpp (A549/ACE2), P = 0.0001 for SARS-2-Spp versus VSV-Gpp (293 T/ACE2), P = 0.0005 for SARS-2-Spp versus VSV-Gpp (Caco-2), P = 0.0002 for SARS-2-Spp versus VSV-Gpp (Huh-7) by two-tailed Student’s t-tests. Animal and cell-based studies were performed independently at least three times with comparable results. The data were the means ± SEMs. **P < 0.01; ***P < 0.001.
Extended Data Fig. 10 Hypothetical model of the involvement of GP73 in SARS-CoV-2-induced metabolic disorder.
Acute SARS-CoV-2 infection increases the cellular secretion of GP73 via the N and/or S protein. Secreted GP73 traffics to the liver to stimulate gluconeogenesis, leading to hyperglycemia. Sustained GP73 elevation via unknown stimuli may directly predispose the host to metabolic disorders. Targeting GP73 may have therapeutic potential for the treatment of patients with this altered hormone during insulin resistance. The figure was created and exported with BioRender.com under a paid subscription.
Supplementary information
Supplementary Information
Supplementary Tables
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig.5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 9
Statistical Source Data.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Wan, L., Gao, Q., Deng, Y. et al. GP73 is a glucogenic hormone contributing to SARS-CoV-2-induced hyperglycemia. Nat Metab 4, 29–43 (2022). https://doi.org/10.1038/s42255-021-00508-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-021-00508-2
This article is cited by
-
Covid 19 and diabetes in children: advances and strategies
Diabetology & Metabolic Syndrome (2024)
-
PTEN deficiency potentiates HBV-associated liver cancer development through augmented GP73/GOLM1
Journal of Translational Medicine (2024)
-
Deficiency of ASGR1 promotes liver injury by increasing GP73-mediated hepatic endoplasmic reticulum stress
Nature Communications (2024)
-
Severe COVID-19 associated hyperglycemia is caused by beta cell dysfunction: a prospective cohort study
Nutrition & Diabetes (2023)
-
Does COVID-19 Infection Increase the Risk of Diabetes? Current Evidence
Current Diabetes Reports (2023)