Abstract
TUG tethering proteins bind and sequester GLUT4 glucose transporters intracellularly, and insulin stimulates TUG cleavage to translocate GLUT4 to the cell surface and increase glucose uptake. This effect of insulin is independent of phosphatidylinositol 3-kinase, and its physiological relevance remains uncertain. Here we show that this TUG cleavage pathway regulates both insulin-stimulated glucose uptake in muscle and organism-level energy expenditure. Using mice with muscle-specific Tug (Aspscr1)-knockout and muscle-specific constitutive TUG cleavage, we show that, after GLUT4 release, the TUG C-terminal cleavage product enters the nucleus, binds peroxisome proliferator-activated receptor (PPAR)γ and its coactivator PGC-1α and regulates gene expression to promote lipid oxidation and thermogenesis. This pathway acts in muscle and adipose cells to upregulate sarcolipin and uncoupling protein 1 (UCP1), respectively. The PPARγ2 Pro12Ala polymorphism, which reduces diabetes risk, enhances TUG binding. The ATE1 arginyltransferase, which mediates a specific protein degradation pathway and controls thermogenesis, regulates the stability of the TUG product. We conclude that insulin-stimulated TUG cleavage coordinates whole-body energy expenditure with glucose uptake, that this mechanism might contribute to the thermic effect of food and that its attenuation could promote obesity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data are available in the manuscript or extended data or in the associated source data files. Additional source data are available from the corresponding author upon reasonable request. RNA-seq data were deposited in Gene Expression Omnibus under the accession number GSE134846. Source data are provided with this paper.
References
Bogan, J. S. Regulation of glucose transporter translocation in health and diabetes. Annu. Rev. Biochem. 81, 507–532 (2012).
Leto, D. & Saltiel, A. R. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 13, 383–396 (2012).
Xu, Y., Nan, D., Fan, J., Bogan, J. S. & Toomre, D. Optogenetic activation reveals distinct roles of PIP3 and Akt in adipocyte insulin action. J. Cell Sci. 129, 2085–2095 (2016).
Czech, M. P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 23, 804–814 (2017).
Habtemichael, E. N. et al. Usp25m protease regulates ubiquitin-like processing of TUG proteins to control GLUT4 glucose transporter translocation in adipocytes. J. Biol. Chem. 293, 10466–10486 (2018).
Govers, R., Coster, A. C. & James, D. E. Insulin increases cell surface GLUT4 levels by dose dependently discharging GLUT4 into a cell surface recycling pathway. Mol. Cell. Biol. 24, 6456–6466 (2004).
Xu, Y. et al. Dual-mode of insulin action controls GLUT4 vesicle exocytosis. J. Cell Biol. 193, 643–653 (2011).
Yu, C., Cresswell, J., Loffler, M. G. & Bogan, J. S. The glucose transporter 4-regulating protein TUG is essential for highly insulin-responsive glucose uptake in 3T3-L1 adipocytes. J. Biol. Chem. 282, 7710–7722 (2007).
Garvey, W. T. et al. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J. Clin. Invest. 101, 2377–2386 (1998).
Maianu, L., Keller, S. R. & Garvey, W. T. Adipocytes exhibit abnormal subcellular distribution and translocation of vesicles containing glucose transporter 4 and insulin-regulated aminopeptidase in type 2 diabetes mellitus: implications regarding defects in vesicle trafficking. J. Clin. Endocrinol. Metab. 86, 5450–5456 (2001).
Li, D. T. et al. GLUT4 storage vesicles: specialized organelles for regulated trafficking. Yale J. Biol. Med. 92, 453–470 (2019).
Bogan, J. S., Hendon, N., McKee, A. E., Tsao, T. S. & Lodish, H. F. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature 425, 727–733 (2003).
Belman, J. P., Habtemichael, E. N. & Bogan, J. S. A proteolytic pathway that controls glucose uptake in fat and muscle. Rev. Endocr. Metab. Disord. 15, 55–66 (2014).
Habtemichael, E. N. et al. Coordinated regulation of vasopressin inactivation and glucose uptake by action of TUG protein in muscle. J. Biol. Chem. 290, 14454–14461 (2015).
Kandror, K. V. & Pilch, P. F. The sugar is sIRVed: sorting Glut4 and its fellow travelers. Traffic 12, 665–671 (2011).
Bogan, J. S. et al. Endoproteolytic cleavage of TUG protein regulates GLUT4 glucose transporter translocation. J. Biol. Chem. 287, 23932–23947 (2012).
Belman, J. P. et al. Acetylation of TUG protein promotes the accumulation of GLUT4 glucose transporters in an insulin-responsive intracellular compartment. J. Biol. Chem. 290, 4447–4463 (2015).
Löffler, M. G. et al. Enhanced fasting glucose turnover in mice with disrupted action of TUG protein in skeletal muscle. J. Biol. Chem. 288, 20135–20150 (2013).
Cantley, J. L. et al. Targeting sterol receptor coactivator-1 with antisense oligonucleotides increases insulin stimulated skeletal muscle glucose uptake in male rats. Am. J. Physiol. Endocrinol. Metab. 307, E773–E783 (2014).
Semiz, S. et al. Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. EMBO J. 22, 2387–2399 (2003).
Chiang, S. H. et al. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410, 944–948 (2001).
Baumann, C. A. et al. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407, 202–207 (2000).
Chang, L., Chiang, S. H. & Saltiel, A. R. TC10α is required for insulin-stimulated glucose uptake in adipocytes. Endocrinology 148, 27–33 (2007).
JeBailey, L. et al. Skeletal muscle cells and adipocytes differ in their reliance on TC10 and Rac for insulin-induced actin remodeling. Mol. Endocrinol. 18, 359–372 (2004).
Coisy-Quivy, M. et al. TC10 controls human myofibril organization and is activated by the sarcomeric RhoGEF obscurin. J. Cell Sci. 122, 947–956 (2009).
Gupte, A. & Mora, S. Activation of the Cbl insulin signaling pathway in cardiac muscle; dysregulation in obesity and diabetes. Biochem. Biophys. Res. Commun. 342, 751–757 (2006).
Bernard, J. R., Reeder, D. W., Herr, H. J., Rivas, D. A. & Yaspelkis, B. B. 3rd High-fat feeding effects on components of the CAP/Cbl signaling cascade in Sprague-Dawley rat skeletal muscle. Metabolism 55, 203–212 (2006).
Bernard, J. R., Saito, M., Liao, Y. H., Yaspelkis, B. B. 3rd & Ivy, J. L. Exercise training increases components of the c-Cbl-associated protein/c-Cbl signaling cascade in muscle of obese Zucker rats. Metabolism 57, 858–866 (2008).
Hart, Y. & Alon, U. The utility of paradoxical components in biological circuits. Mol. Cell 49, 213–221 (2013).
Li, D. T., Habtemichael, E. N. & Bogan, J. S. Vasopressin inactivation: role of insulin-regulated aminopeptidase. Vitam. Horm. 113, 101–128 (2020).
Schertzer, J. D. et al. A transgenic mouse model to study glucose transporter 4myc regulation in skeletal muscle. Endocrinology 150, 1935–1940 (2009).
Nedergaard, J. & Cannon, B. The browning of white adipose tissue: some burning issues. Cell Metab. 20, 396–407 (2014).
Bal, N. C., Sahoo, S. K., Maurya, S. K. & Periasamy, M. The role of sarcolipin in muscle non-shivering thermogenesis. Front. Physiol. 9, 1217 (2018).
Ikeda, K. et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 23, 1454–1465 (2017).
Reinisch, I., Schreiber, R. & Prokesch, A. Regulation of thermogenic adipocytes during fasting and cold. Mol. Cell. Endocrinol. 512, 110869 (2020).
Orme, C. M. & Bogan, J. S. The ubiquitin regulatory X (UBX) domain-containing protein TUG regulates the p97 ATPase and resides at the endoplasmic reticulum–Golgi intermediate compartment. J. Biol. Chem. 287, 6679–6692 (2012).
Altshuler, D. et al. The common PPARγ Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat. Genet. 26, 76–80 (2000).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Trausch-Azar, J., Leone, T. C., Kelly, D. P. & Schwartz, A. L. Ubiquitin proteasome-dependent degradation of the transcriptional coactivator PGC-1α via the N-terminal pathway. J. Biol. Chem. 285, 40192–40200 (2010).
Adamovich, Y. et al. The protein level of PGC-1α, a key metabolic regulator, is controlled by NADH–NQO1. Mol. Cell. Biol. 33, 2603–2613 (2013).
Sano, M. et al. Intramolecular control of protein stability, subnuclear compartmentalization, and coactivator function of peroxisome proliferator-activated receptor γ coactivator 1α. J. Biol. Chem. 282, 25970–25980 (2007).
Wang, H., Liu, L., Lin, J. Z., Aprahamian, T. R. & Farmer, S. R. Browning of white adipose tissue with roscovitine induces a distinct population of UCP1+ adipocytes. Cell Metab. 24, 835–847 (2016).
Banchenko, S. et al. Common mode of remodeling AAA ATPases p97/CDC48 by their disassembling cofactors ASPL/PUX1. Structure 27, 1830–1841 (2019).
van den Boom, J. & Meyer, H. VCP/p97-mediated unfolding as a principle in protein homeostasis and signaling. Mol. Cell 69, 182–194 (2018).
Varshavsky, A. N-degron and C-degron pathways of protein degradation. Proc. Natl Acad. Sci. USA 116, 358–366 (2019).
Brower, C. S. & Varshavsky, A. Ablation of arginylation in the mouse N-end rule pathway: loss of fat, higher metabolic rate, damaged spermatogenesis, and neurological perturbations. PLoS ONE 4, e7757 (2009).
Cui, J. et al. Adipose-specific deletion of Kif5b exacerbates obesity and insulin resistance in a mouse model of diet-induced obesity. FASEB J. 31, 2533–2547 (2017).
Lowell, B. B. & Spiegelman, B. M. Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 (2000).
Reed, G. W. & Hill, J. O. Measuring the thermic effect of food. Am. J. Clin. Nutr. 63, 164–169 (1996).
Haeusler, R. A., McGraw, T. E. & Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 19, 31–44 (2018).
de Jonge, L. & Bray, G. A. The thermic effect of food is reduced in obesity. Nutr. Rev. 60, 223–233 (2002).
Patti, M. E. et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc. Natl Acad. Sci. USA 100, 8466–8471 (2003).
Finck, B. N. & Kelly, D. P. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 116, 615–622 (2006).
Puigserver, P. et al. Activation of PPARγ coactivator-1 through transcription factor docking. Science 286, 1368–1371 (1999).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
St-Pierre, J. et al. Bioenergetic analysis of peroxisome proliferator-activated receptor γ coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J. Biol. Chem. 278, 26597–26603 (2003).
Choi, C. S. et al. Paradoxical effects of increased expression of PGC-1α on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc. Natl Acad. Sci. USA 105, 19926–19931 (2008).
Summermatter, S., Baum, O., Santos, G., Hoppeler, H. & Handschin, C. Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) promotes skeletal muscle lipid refueling in vivo by activating de novo lipogenesis and the pentose phosphate pathway. J. Biol. Chem. 285, 32793–32800 (2010).
Summermatter, S. et al. PGC-1α improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes 62, 85–95 (2013).
Quan, N. et al. Sestrin2 prevents age-related intolerance to ischemia and reperfusion injury by modulating substrate metabolism. FASEB J. 31, 4153–4167 (2017).
Chen, Y. et al. Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes. J. Cell Biol. 198, 545–560 (2012).
Wasserman, D. H., Kang, L., Ayala, J. E., Fueger, P. T. & Lee-Young, R. S. The physiological regulation of glucose flux into muscle in vivo. J. Exp. Biol. 214, 254–262 (2011).
Hu, R. G. et al. The N-end rule pathway as a nitric oxide sensor controlling the levels of multiple regulators. Nature 437, 981–986 (2005).
Karakozova, M. et al. Arginylation of β-actin regulates actin cytoskeleton and cell motility. Science 313, 192–196 (2006).
Liu, X. et al. Generation of mammalian cells stably expressing multiple genes at predetermined levels. Anal. Biochem. 280, 20–28 (2000).
Tschop, M. H. et al. A guide to analysis of mouse energy metabolism. Nat. Methods 9, 57–63 (2011).
Corrigan, J. K. et al. A big-data approach to understanding metabolic rate and response to obesity in laboratory mice. eLife 9, e53560 (2020).
Camporez, J. P. et al. Mechanism by which arylamine N-acetyltransferase 1 ablation causes insulin resistance in mice. Proc. Natl Acad. Sci. USA 114, E11285–E11292 (2017).
Camporez, J. P. et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology 154, 1021–1028 (2013).
Choi, C. S. et al. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc. Natl Acad. Sci. USA 104, 16480–16485 (2007).
Vatner, D. F. et al. Thyroid hormone receptor-β agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am. J. Physiol. Endocrinol. Metab. 305, E89–E100 (2013).
Chaudry, I. H. & Gould, M. K. Kinetics of glucose uptake in isolated soleus muscle. Biochim. Biophys. Acta 177, 527–536 (1969).
Cuendet, G. S., Loten, E. G., Jeanrenaud, B. & Renold, A. E. Decreased basal, noninsulin-stimulated glucose uptake and metabolism by skeletal soleus muscle isolated from obese-hyperglycemic (ob/ob) mice. J. Clin. Invest. 58, 1078–1088 (1976).
Wang, C., Yue, F. & Kuang, S. Muscle histology characterization using H&E staining and muscle fiber type classification using immunofluorescence staining. Bio-protocol 7, e2279 (2017).
Bloemberg, D. & Quadrilatero, J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 7, e35273 (2012).
Hauser, S. et al. Degradation of the peroxisome proliferator-activated receptor γ is linked to ligand-dependent activation. J. Biol. Chem. 275, 18527–18533 (2000).
Lin, J., Puigserver, P., Donovan, J., Tarr, P. & Spiegelman, B. M. Peroxisome proliferator-activated receptor γ coactivator 1β (PGC-1β), a novel PGC-1-related transcription coactivator associated with host cell factor. J. Biol. Chem. 277, 1645–1648 (2002).
Monsalve, M. et al. Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol. Cell 6, 307–316 (2000).
Kim, J. H. et al. Suppression of PPARγ through MKRN1-mediated ubiquitination and degradation prevents adipocyte differentiation. Cell Death Differ. 21, 594–603 (2014).
Kamitani, T., Kito, K., Nguyen, H. P. & Yeh, E. T. Characterization of NEDD8, a developmentally down-regulated ubiquitin-like protein. J. Biol. Chem. 272, 28557–28562 (1997).
Yoo, J., Kohlbrenner, E., Kim, O., Hajjar, R. J. & Jeong, D. Enhancing atrial-specific gene expression using a calsequestrin cis-regulatory module 4 with a sarcolipin promoter. J. Gene Med. 20, e3060 (2018).
Bogan, J. S., McKee, A. E. & Lodish, H. F. Insulin-responsive compartments containing GLUT4 in 3T3-L1 and CHO cells: regulation by amino acid concentrations. Mol. Cell. Biol. 21, 4785–4806 (2001).
Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: discovering splice junctions with RNA-seq. Bioinformatics 25, 1105–1111 (2009).
Trapnell, C. et al. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Eden, E., Navon, R., Steinfeld, I., Lipson, D. & Yakhini, Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10, 48 (2009).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Chen, X. & Prywes, R. Serum-induced expression of the cdc25A gene by relief of E2F-mediated repression. Mol. Cell. Biol. 19, 4695–4702 (1999).
Acknowledgements
We thank M. G. Löffler, A. Alcázar-Román, O. Julca-Zevallos, J. Culver, E. Zagoren, S. Mane, W. H. Brown, E. Li, R. Calia-Bogan, A. Kashina, D. Jeong and D. Toomre for advice, reagents and assistance. This work used the Core Facilities of the Yale Diabetes Research Center (DRC, NIH P30 DK045735) and services of the CCMI EM facility and the Keck Biotechnology Resource at Yale University. This work was supported by NIH R01 DK092661 and R56 DK092661 (to J.S.B.), by the American Diabetes Association 1-17-IBS-40 (to J.S.B.) and by R01 DK116774 and R01 DK114793 (to G.I.S.). D.T.L. was supported by T32 GM136651 and F30 DK115037; E.N.H. was supported by a Yale DRC Pilot grant under P30 DK045735; and D.F.V. was supported by R01 DK124272. L.G. was supported by K99 HL150234. J.P.C. was supported by the São Paulo Research Foundation (FAPESP), grant 2018/04956-5. H.L. was supported by the Natural Science Foundation of Zhejiang Province, China, no. LY18H070004. S.H. was supported by the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES/PVEX-88881.170862/2018-01) and the Postgraduate and Research Dean Award, Cruzeiro do Sul (PRPGP/UNICSUL-0708/2019).
Author information
Authors and Affiliations
Contributions
E.N.H., D.T.L. and J.S.B. conceptualised the project and designed the experiments. E.N.H., D.T.L., J.P.C., X.O.W., C.I.S., X.L., F.L.-G., S.G.D., H.L., D.M.R., K.Y.W., B.S.S., S.G.Z., P.D., S.N.B., S.H., D.F.V., L.G., W.P., G.I.S. and J.S.B. performed experiments and analysed data. J.S.B. supervised the overall project, and D.T.L. and J.S.B. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Metabolism thanks Shingo Kajimura and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Christoph Schmitt.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of glucose homeostasis in MTKO mice.
a,b, Relative TUG abundance was quantified in quadriceps (a) and heart (b) using densitometry of immunoblots of tissues from 12-week old MTKO and WT control mice. N=5 in each group. c, Quadriceps muscles from fasted MTKO and WT mice were homogenized and T-tubule -enriched membrane fractions were purified and immunoblotted, as indicated. The first three samples in each group are the same as the unstimulated samples shown in Fig. 1c. All four unstimulated samples shown here were included in the quantification shown in Fig. 1d,e. d, Control immunoblots of T-tubule fractions and total homogenates from quadriceps of fasted mice were done as indicated to demonstrate the purity of the fractions. e, The relative abundance of intact TUG in quadriceps of WT mice was quantified by densitometry of the immunoblot in Fig. 1c. N=3 in each group. f–h, Body weights and composition were measured in 17-week old WT and MTKO mice. N=8 in each group. i, Heart weights were measured in 12-week old WT and MTKO mice. N=9 WT, 8 MTKO mice. j, Fasting glucose concentrations were measured in blood obtained by cardiac puncture of 16-week old mice. N=9 WT and 11 MTKO mice. k, HOMA-IR was calculated from paired insulin and glucose measurements plotted individually in Fig. 1g and in (j), in 4–6 h fasted 16-week old mice. N=5 in each group. l, Basal plasma glucose was measured prior to turnover studies in fasting 19-week old mice. N=8 WT and 9 MKTO mice. m, Heart-specific glucose uptake was measured in fasting 19-week old mice. N=8 WT and 9 MKTO mice. All data are presented as mean ± SEM of biologically independent samples, analyzed using two-tailed t-tests.
Extended Data Fig. 2 Characterization of energy expenditure in MTKO mice.
a–d, The indicated parameters were measured in 17-week old WT and MTKO mice in metabolic cages. Energy expenditure was normalized to total weight (c) and lean body mass (LBM; d). N=8 WT and 7 MTKO mice. e–g, Energy expenditure measurements are plotted vs. time of day (e) and during light (f) and dark (g) hours. N=8 WT and 7 MTKO mice. h–j, Respiratory exchange ratio (RER; h), locomotor activity (i), and food intake (j) are plotted. N=8 WT and 7 MTKO mice. k, Water intake was measured in 22-week old WT and MTKO mice in metabolic cages. N=16 in each group. All data are presented as mean ± SEM of biologically independent samples, analyzed using two-tailed t-tests.
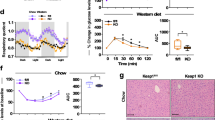
Extended Data Fig. 3 MTKO mice are susceptible to obesity and have reduced energy expenditure on a high-fat diet.
a, Mice were fed a high-fat diet (HFD) beginning at 15 weeks of age, and the percentage weight gain from baseline is plotted. N=10 WT and 12 MTKO mice. b, After the HFD, HOMA-IR was calculated from paired measurements of plasma glucose and insulin. N=7 WT and 10 MTKO mice. c, Immunoblots were done as indicated on hindlimb muscles from mice fed a HFD for 3 weeks. d–f, Body weights and composition of 14-week old MTKO and WT mice fed a HFD for 3 weeks prior to measurements done in metabolic cages. N=7 in each group. g–q, HFD-fed 14-week old MTKO and WT mice were housed in metabolic cages and the indicated parameters were measured. N=7 in each group (g–l, p, q); N=7 WT and 6 MTKO mice (m–o). RER, respiratory exchange ratio; LBM, lean body mass. All data are presented as mean ± SEM of biologically independent samples, analyzed using two-tailed t-tests. *p<0.05, **p<0.01, ***p<0.001.
Extended Data Fig. 4 MTKO mice have increased weight gain and fat mass when housed at thermoneutrality.
a, Body weights were measured in male MTKO and WT mice housed at 30 °C from weaning. All mice were maintained on regular chow. N = 11 WT and 9 MTKO mice. b, Body weights were measured in female MTKO and WT mice housed at 30 °C from weaning. All mice were maintained on regular chow. N = 12 WT and 8 MTKO mice. c,d, Gonadal white adipose tissues from 20-week old mice used for data in (a) and (b) were weighed. N=5 WT and 3 MTKO males, and 10 WT and 5 MTKO females. All data are presented as mean ± SEM of biologically independent samples, analyzed using two-tailed t-tests. *p<0.05, **p<0.01, ***p<0.001.
Extended Data Fig. 5 Expression of specific genes involved in energy expenditure is reduced in MTKO mice and increased in UBX mice.
a–e, RNA was prepared from quadriceps muscles of ad lib fed 10-week old WT, MTKO, and UBX mice, and qPCR was used to measure the relative abundances of selected transcripts. N=4 WT, 5 MTKO, and 4 UBX mice except for N=3 for UBX TUG-Cter data (a). a, Control reactions were used to verify knockout of TUG in MTKO mice and expression of the UBX-Cter transgene in UBX mice. b, Relative abundances of transcripts for Sarcolipin (Sln), Ucp1, and β3-adrenergic receptor (Adrb3) are shown. c, Relative abundances of the indicated transcripts involved in energy expenditure and lipid metabolism are shown. d, Relative abundances of transcripts encoding PGC-1α (Ppargc1a) and PPARγ (Pparg) are shown. e, Relative abundances of transcripts encoding the indicated calsequestrin proteins are shown. Data are presented as mean ± SEM of biologically independent samples, analyzed using two-tailed t-tests (a–e). f, Gene set enrichment analysis was done on transcripts that were differentially expressed in quadriceps of 11-week old, 4-6 h fasted UBX mice, compared to WT controls (N=3 mice in each group; transcripts were analyzed using CuffDiff as described in the Methods section). Transcripts were ranked in order of significance, and the top 2000 transcripts were analyzed using the GO Biological Process ontology gene set. The gene set corresponding to “Temperature Homeostasis” was significantly enriched (Fig. 3b; False Discovery Rate q value = 0.038)). Here, specific genes in this set that were differentially expressed in UBX vs. WT muscles are listed, together with their rank in the gene list, the fold-change in expression in UBX muscles compared to controls, the adjusted p-value in the RNA-seq data set (text is bold if p<0.05 after adjustment for multiple comparisons using the Benjamini method), and the running enrichment score calculated by GSEA software.
Extended Data Fig. 6 The TUG C-terminal product enters the nucleus and binds PPARγ and PGC-1α.
a, Nuclear fractions were prepared from quadriceps of WT, UBX, and MTKO mice that had been fasted, treated with IP injection of insulin-glucose solution or saline control, and euthanized 30 min. after injection. Immunoblots were done as indicated. b,c, Proteins were expressed by transient transfection of HEK293 cells, and immunoprecipitations (IP) and western blots (WB) were performed, as indicated. d, Recombinant proteins were produced as GST fusions, immobilized on glutathione beads, and incubated with recombinant TUG C-terminal cleavage product (residues 165-550). Bound TUG protein was eluted and western blots were performed as indicated. e, Recombinant proteins were immobilized and incubated with soluble recombinant PPARγ2 protein. Bound PPARγ2 was eluted and western blots were performed as indicated. f, Truncated forms of TUG were produced as GST fusions and the GST was cleaved off to yield soluble TUG fragments. These were incubated with immobilized GST, PGC-1α, and PPARγ2 as indicated. Bound proteins were eluted and immunoblotted as indicated. g, Peptides corresponding to the 37 residues at the N-termini of PPARγ1 or PPARγ2 were immobilized on streptavidin beads. The TUG C-terminal product (beginning with a Met residue) was expressed stably in MEFs using a retrovirus, and lysates from these cells were incubated with the beads. Bound proteins were eluted and immunoblotted. h, Peptides corresponding to the 29 N-terminal residues of PPARγ2, containing Pro12 or Ala12 residues, were immobilized on beads and incubated with lysates of MEFs expressing the TUG C-terminal product. Bound proteins were eluted and immunoblotted as indicated. i, Peptides used in (h) were incubated with HEK293 lysates, and bound endogenous (human) intact TUG was eluted and immunoblotted. j, WT and MTKO mice were treated by IP injection of insulin-glucose solution, then euthanized after 3 h. PGC-1α was immunoprecipitated from lysates of quadriceps, and immunoblots were done to detect bound PPARγ, as in Fig. 3g. The relative abundances of PPARγ in replicate experiments were quantified. N=3 biologically independent samples, presented as mean ±SEM, analyzed using a two-tailed t-test.
Extended Data Fig. 7 TUG controls PGC-1α protein abundance.
a, WT and MTKO mice were treated by IP injection of insulin-glucose, or saline control, then sacrificed at the indicated times after injection. Quadriceps muscles were immunoblotted as indicated. b,c, WT and MTKO mice were treated by IP injection of insulin-glucose, or saline control, then sacrificed after 3 h. Lysates were prepared from quadriceps muscles, PGC-1α was immunoblotted, and the relative abundances in each sample were quantified using densitometry. Data in (c) are presented as mean ±SEM of biologically independent samples (N=3 in each group), analyzed using ANOVA with adjustment for multiple comparisons. d, WT and MTKO mice were treated with IP insulin-glucose, or saline control, then sacrificed 3 h later. RNA was prepared from quadriceps muscles, and Q-PCR was used to measure PGC-1α (Ppargc1a) mRNA abundance. Data are presented as mean ±SEM of biologically independent samples (N=3 in each group), analyzed using ANOVA with adjustment for multiple comparisons.
Extended Data Fig. 8 TUG regulates mitochondrial function and morphology and has no large effect on muscle fiber type.
a, WT and MTKO soleus muscles from mice that had been fed a HFD for 2.5 weeks were imaged using electron microscopy. Lipid droplets were noted in MTKO muscles, but not WT muscles, and were adjacent to mitochondria, as shown. b–e, Images of soleus muscles from HFD-fed WT and MTKO mice (N=3 each) were obtained by electron microscopy and were analyzed to quantify mitochondrial density (b), area (c), length (d), and width (e). Mitochondria were traced manually on 5-9 images from each mouse. Each data point represents the average of the measurements from a single image. N=22 WT and 21 MTKO images were quantified. Data are presented as mean ±SEM and analyzed using two-tailed t-tests. f, Relative abundances of the indicated transcripts in quadriceps muscles were measured using qPCR. N=4 WT, 5 MTKO, and 4 UBX mice. Data are plotted as mean ±SEM and pairwise analyses were done using two-tailed t-tests. g, Cross-sections of quadriceps muscles were stained to detect myosin heavy chain type IIA using immunohistochemistry. h, The percentage of muscle fibers that stained for MHC type IIA was quantified from 2-3 images from each of 5 separate mice of each genotype. N=12 WT, 12 MTKO, and 14 UBX images were quantified, and data are plotted as mean ±SEM.
Extended Data Fig. 9 Sarcolipin abundance is regulated by TUG and is decreased in diet-induced insulin resistance.
a, WT and MTKO mice were housed at 30 °C from the time of weaning. At age 20 weeks, mice were fasted, treated with IP insulin-glucose or saline control, and sacrificed 3 h later. Hindlimb muscle lysates were immunoblotted as indicated. Data from replicates of this experiment were quantified and are plotted in Fig. 4b. b, WT and UBX mice were treated with IP insulin-glucose, or saline control, sacrificed after 30 min., and quadriceps muscles were immunoblotted as indicated. c,d, WT and MTKO mice were treated with IP insulin-glucose, or saline control, and sacrificed after 30 min. Hindlimb muscles were used for chromatin immunoprecipitation using PGC-1α (c) and PPARγ (d) antibodies, as indicated. PCR was used to detect an amplicon at -200 nucleotides relative to the sarcolipin transcription start site. e, WT mice were fed regular chow (RC) or a high-fat diet (HFD) for 3 weeks, then treated for 30 min. with IP insulin-glucose, or saline control. Quadriceps lysates were immunoblotted to detect intact TUG and the C-terminal cleavage product. Replicates were quantified using densitometry and are plotted in Fig. 4f. f, WT mice were fed RC or a HFD for 3 weeks, fasted, and sacrificed. Hindlimb muscles were isolated and immunoblotted as indicated. Replicates were quantified using densitometry and are plotted in Fig. 4g.
Supplementary information
Supplementary Information
Supplementary Tables 2 and 3.
Supplementary Table 1
Differentially expressed transcripts in quadriceps of UBX versus WT mice.
Source data
Source Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 7
Unprocessed western blots and/or gels.
Rights and permissions
About this article
Cite this article
Habtemichael, E.N., Li, D.T., Camporez, J.P. et al. Insulin-stimulated endoproteolytic TUG cleavage links energy expenditure with glucose uptake. Nat Metab 3, 378–393 (2021). https://doi.org/10.1038/s42255-021-00359-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-021-00359-x
This article is cited by
-
Endosomal trafficking in metabolic homeostasis and diseases
Nature Reviews Endocrinology (2023)
-
Insulin action and resistance are dependent on a GSK3β-FBXW7-ERRα transcriptional axis
Nature Communications (2022)
-
Inhibitors of RNA and protein synthesis cause Glut4 translocation and increase glucose uptake in adipocytes
Scientific Reports (2022)