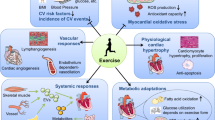
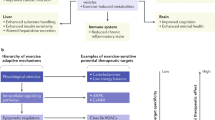
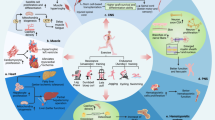
Abstract
The cardiac benefits of exercise have been recognized for centuries. Studies have undisputedly shown that regular exercise is beneficial for the cardiovascular system in young, old, healthy and diseased populations. For these reasons, physical activity has been recommended worldwide for cardiovascular disease prevention and treatment. Although the benefits of exercise are clear, understanding of the molecular triggers that orchestrate these effects remains incomplete and has been a topic of intense research in recent years. Here, we provide a comprehensive review of the cardiac effects of physical activity, beginning with a brief history of exercise in cardiovascular medicine and then discussing seminal work on the physiological effects of exercise in healthy, diseased and aged hearts. Later, we revisit pioneering work on the molecular mechanisms underlying the cardiac benefits of exercise, and we conclude with our view on the translational potential of this knowledge as a powerful platform for cardiovascular disease drug discovery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
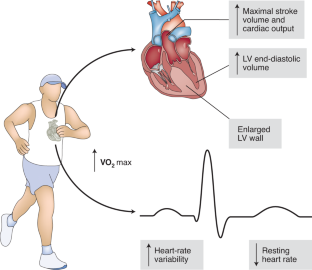
Similar content being viewed by others
References
Ross, R. et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 134, e653–e699 (2016).
Laukkanen, J. A. et al. Cardiovascular fitness as a predictor of mortality in men. Arch. Intern. Med. 161, 825–831 (2001).
Lavie, C. J. & Milani, R. V. Effects of cardiac rehabilitation, exercise training, and weight reduction on exercise capacity, coronary risk factors, behavioral characteristics, and quality of life in obese coronary patients. Am. J. Cardiol. 79, 397–401 (1997).
Marchionni, N. et al. Improved exercise tolerance and quality of life with cardiac rehabilitation of older patients after myocardial infarction: results of a randomized, controlled trial. Circulation 107, 2201–2206 (2003).
Roh, J., Rhee, J., Chaudhari, V. & Rosenzweig, A. The role of exercise in cardiac aging: from physiology to molecular mechanisms. Circ. Res. 118, 279–295 (2016).
Wisløff, U., Helgerud, J., Kemi, O. J. & Ellingsen, O. Intensity-controlled treadmill running in rats: VO2 max and cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 280, H1301–H1310 (2001).
Wisløff, U. et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115, 3086–3094 (2007).
Ramazzini, B. De morbis artificum diatriba [Diseases of workers]. 1713. Am. J. Public Health 91, 1380–1382 (2001).
Hartley, P. H. & Llewellyn, G. F. Longevity of oarsmen. BMJ 1, 657–662 (1939).
Morris, J. N., Heady, J. A., Raffle, P. A., Roberts, C. G. & Parks, J. W. Coronary heart-disease and physical activity of work. Lancet 262, 1053–1057 (1953).
Blair, S. N., Cheng, Y. & Holder, J. S. Is physical activity or physical fitness more important in defining health benefits? Med. Sci. Sports Exerc. 33, S379–399 (2001).
Dunn, A. L. et al. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. J. Am. Med. Assoc. 281, 327–334 (1999).
Blair, S. N. et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. J. Am. Med. Assoc. 276, 205–210 (1996).
Lee, D. C. et al. Leisure-time running reduces all-cause and cardiovascular mortality risk. J. Am. Coll. Cardiol. 64, 472–481 (2014).
Ortega, F. B., Ruiz, J. R., Castillo, M. J. & Sjostrom, M. Physical fitness in childhood and adolescence: a powerful marker of health. Int. J. Obes. 32, 1–11 (2008).
Lee, I. M., Hsieh, C. C. & Paffenbarger, R. S. Jr. Exercise intensity and longevity in men: The Harvard Alumni Health Study. J. Am. Med. Assoc. 273, 1179–1184 (1995).
Wei, M. et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. J. Am. Med. Assoc. 282, 1547–1553 (1999).
Saltin, B. et al. Response to exercise after bed rest and after training. Circulation 38, 1–78 (1968). The article describes changes in VO2 max and cardiac variables as a result of 20 days of bed rest followed by 8 weeks of exercise training.
Shephard, R. J. et al. The maximum oxygen intake: an international reference standard of cardiorespiratory fitness. Bull. World Health Organ. 38, 757–764 (1968).
Wagner, P. D. Determinants of maximal oxygen transport and utilization. Annu. Rev. Physiol. 58, 21–50 (1996).
Arbab-Zadeh, A. et al. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation 130, 2152–2161 (2014).
Zavorsky, G. S. Evidence and possible mechanisms of altered maximum heart rate with endurance training and tapering. Sports Med. 29, 13–26 (2000).
Pombo, J. F., Troy, B. L. & Russell, R. O. Jr. Left ventricular volumes and ejection fraction by echocardiography. Circulation 43, 480–490 (1971).
Morganroth, J., Maron, B. J., Henry, W. L. & Epstein, S. E. Comparative left ventricular dimensions in trained athletes. Ann. Intern. Med. 82, 521–524 (1975).
Fagard, R. H. Athlete’s heart: a meta-analysis of the echocardiographic experience. Int. J. Sports Med. 17 (Suppl. 3), S140–S144 (1996).
Spence, A. L. et al. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J. Physiol. (Lond.) 589, 5443–5452 (2011).
McMullen, J. R. & Jennings, G. L. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin. Exp. Pharmacol. Physiol. 34, 255–262 (2007).
Lovic, D. et al. Left ventricular hypertrophy in athletes and hypertensive patients. J. Clin. Hypertens. (Greenwich) 19, 413–417 (2017).
Iwasaki, K., Zhang, R., Zuckerman, J. H. & Levine, B. D. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J. Appl. Physiol. 95, 1575–1583 (2003).
Carter, J. B., Banister, E. W. & Blaber, A. P. Effect of endurance exercise on autonomic control of heart rate. Sports Med. 33, 33–46 (2003).
Sidhu, S. & Marine, J. E. Evaluating and managing bradycardia. Trends Cardiovasc. Med. 30, 265–272 (2020).
Saito, Y. et al. HCN4-overexpressing mouse embryonic stem cell-derived cardiomyocytes generate a new rapid rhythm in rats with bradycardia. Int. Heart J. 59, 601–606 (2018).
Nof, E., Antzelevitch, C. & Glikson, M. The contribution of HCN4 to normal sinus node function in humans and animal models. Pacing Clin. Electrophysiol. 33, 100–106 (2010).
Sneddon, J. F. & Camm, A. J. Sinus node disease: current concepts in diagnosis and therapy. Drugs 44, 728–737 (1992).
Levy, W. C. et al. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am. J. Cardiol. 82, 1236–1241 (1998).
Melanson, E. L. & Freedson, P. S. The effect of endurance training on resting heart rate variability in sedentary adult males. Eur. J. Appl. Physiol. 85, 442–449 (2001).
Carter, J. B., Banister, E. W. & Blaber, A. P. The effect of age and gender on heart rate variability after endurance training. Med. Sci. Sports Exerc. 35, 1333–1340 (2003).
Zingman, L. V. et al. Exercise-induced expression of cardiac ATP-sensitive potassium channels promotes action potential shortening and energy conservation. J. Mol. Cell. Cardiol. 51, 72–81 (2011).
Fletcher, P. J., Pfeffer, J. M., Pfeffer, M. A. & Braunwald, E. Left ventricular diastolic pressure-volume relations in rats with healed myocardial infarction: effects on systolic function. Circ. Res. 49, 618–626 (1981).
Matsuda, Y. et al. Importance of left atrial function in patients with myocardial infarction. Circulation 67, 566–571 (1983).
Tanaka, M. et al. Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy. Br. Heart J. 55, 575–581 (1986).
Konhilas, J. P. et al. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ. Res. 98, 540–548 (2006).
De Angelis, K. et al. Exercise training changes autonomic cardiovascular balance in mice. J. Appl. Physiol. 96, 2174–2178 (2004).
Wisløff, U., Loennechen, J. P., Currie, S., Smith, G. L. & Ellingsen, Ø. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc. Res. 54, 162–174 (2002).
Kemi, O. J. et al. Exercise training corrects control of spontaneous calcium waves in hearts from myocardial infarction heart failure rats. J. Cell. Physiol. 227, 20–26 (2012).
Qin, R. et al. Exercise training reduces ventricular arrhythmias through restoring calcium handling and sympathetic tone in myocardial infarction mice. Physiol. Rep. 7, e13972 (2019).
Malmo, V. et al. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation 133, 466–473 (2016).
Tjønna, A. E. et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation 118, 346–354 (2008).
Hollekim-Strand, S. M. et al. High-intensity interval exercise effectively improves cardiac function in patients with type 2 diabetes mellitus and diastolic dysfunction: a randomized controlled trial. J. Am. Coll. Cardiol. 64, 1758–1760 (2014).
Kong, P., Christia, P. & Frangogiannis, N. G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 71, 549–574 (2014).
Weeks, K. L. et al. Phosphoinositide 3-kinase p110α is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ. Heart Fail 5, 523–534 (2012).
Ma, X. et al. Cardiac fibrosis alleviated by exercise training is AMPK-dependent. PLoS ONE 10, e0129971 (2015).
Puhl, S. L. et al. Exercise attenuates inflammation and limits scar thinning after myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 309, H345–H359 (2015).
Wilhelm, M. J. Long-term outcome following heart transplantation: current perspective. J. Thorac. Dis. 7, 549–551 (2015).
Squires, R. W. Exercise training after cardiac transplantation. Med. Sci. Sports Exerc. 23, 686–694 (1991).
Tegtbur, U., Busse, M. W., Jung, K., Pethig, K. & Haverich, A. Time course of physical reconditioning during exercise rehabilitation late after heart transplantation. J. Heart Lung Transplant. 24, 270–274 (2005).
Karapolat, H. et al. Comparison of hospital-supervised exercise versus home-based exercise in patients after orthotopic heart transplantation: effects on functional capacity, quality of life, and psychological symptoms. Transplant. Proc. 39, 1586–1588 (2007).
Squires, R. W. et al. Partial normalization of the heart rate response to exercise after cardiac transplantation: frequency and relationship to exercise capacity. Mayo Clin. Proc. 77, 1295–1300 (2002).
Bowles, D. K. & Starnes, J. W. Exercise training improves metabolic response after ischemia in isolated working rat heart. J. Appl. Physiol. 76, 1608–1614 (1994).
French, J. P. et al. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J. 22, 2862–2871 (2008).
Powers, S. K. et al. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am. J. Physiol. 275, R1468–R1477 (1998).
Yamashita, N. et al. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J. Exp. Med. 189, 1699–1706 (1999).
Ejlersen, H. et al. Prognostic impact of physical activity prior to myocardial infarction: Case fatality and subsequent risk of heart failure and death. Eur. J. Prev. Cardiol. 24, 1112–1119 (2017).
Peytz, N. C. et al. Physical activity and risk of instant and 28-day case-fatality in myocardial infarction. PLoS ONE 14, e0217398 (2019).
Brandfonbrener, M., Landowne, M. & Shock, N. W. Changes in cardiac output with age. Circulation 12, 557–566 (1955).
Lauer, M. S. et al. Impaired chronotropic response to exercise stress testing as a predictor of mortality. J. Am. Med. Assoc. 281, 524–529 (1999).
Leier, C. V., Heban, P. T., Huss, P., Bush, C. A. & Lewis, R. P. Comparative systemic and regional hemodynamic effects of dopamine and dobutamine in patients with cardiomyopathic heart failure. Circulation 58, 466–475 (1978).
Kappagoda, T. & Amsterdam, E. A. Exercise and heart failure in the elderly. Heart Fail. Rev. 17, 635–662 (2012).
Guarnieri, T., Filburn, C. R., Zitnik, G., Roth, G. S. & Lakatta, E. G. Contractile and biochemical correlates of beta-adrenergic stimulation of the aged heart. Am. J. Physiol. 239, H501–H508 (1980).
Zwiren, L. D., Freedson, P. S., Ward, A., Wilke, S. & Rippe, J. M. Estimation of VO2max: a comparative analysis of five exercise tests. Res. Q. Exerc. Sport 62, 73–78 (1991).
Lambert, M. I. & Noakes, T. D. Spontaneous running increases VO2max and running performance in rats. J. Appl. Phyisiol. 68, 400–403 (1990).
Leosco, D. et al. Exercise training and beta-blocker treatment ameliorate age-dependent impairment of beta-adrenergic receptor signaling and enhance cardiac responsiveness to adrenergic stimulation. Am. J. Physiol. Heart Circ. Physiol. 293, H1596–H1603 (2007).
Böhm, M. et al. Effects of exercise on myocardial adenylate cyclase and Gi alpha expression in senescence. Am. J. Physiol. 264, H805–H814 (1993).
Scarpace, P. J., Shu, Y. & Tumer, N. Influence of exercise training on myocardial beta-adrenergic signal transduction: differential regulation with age. J. Appl. Phyisiol. 77, 737–741 (1994).
Bers, D. M. Cardiac excitation-contraction coupling. Nature 415, 198–205 (2002).
Lim, C. C., Apstein, C. S., Colucci, W. S. & Liao, R. Impaired cell shortening and relengthening with increased pacing frequency are intrinsic to the senescent mouse cardiomyocyte. J. Mol. Cell. Cardiol. 32, 2075–2082 (2000).
Isenberg, G., Borschke, B. & Rueckschloss, U. Ca2+ transients of cardiomyocytes from senescent mice peak late and decay slowly. Cell Calcium 34, 271–280 (2003).
Hamilton, S. & Terentyev, D. Altered intracellular calcium homeostasis and arrhythmogenesis in the aged heart. Int. J. Mol. Sci. 20, E2386 (2019).
Schmidt, U. et al. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca2+-ATPase. Circulation 101, 790–796 (2000).
Tate, C. A. et al. SERCA2a and mitochondrial cytochrome oxidase expression are increased in hearts of exercise-trained old rats. Am. J. Physiol. 271, H68–H72 (1996).
Iemitsu, M. et al. Exercise training improves cardiac function-related gene levels through thyroid hormone receptor signaling in aged rats. Am. J. Physiol. Heart Circ. Physiol. 286, H1696–H1705 (2004).
Biernacka, A. & Frangogiannis, N. G. Aging and cardiac fibrosis. Aging Dis. 2, 158–173 (2011).
Olivetti, G., Melissari, M., Capasso, J. M. & Anversa, P. Cardiomyopathy of the aging human heart: myocyte loss and reactive cellular hypertrophy. Circ. Res. 68, 1560–1568 (1991).
Kwak, H. B. et al. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J. 25, 1106–1117 (2011).
Thomas, D. P., Cotter, T. A., Li, X., McCormick, R. J. & Gosselin, L. E. Exercise training attenuates aging-associated increases in collagen and collagen crosslinking of the left but not the right ventricle in the rat. Eur. J. Appl. Physiol. 85, 164–169 (2001).
Arbab-Zadeh, A. et al. Effect of aging and physical activity on left ventricular compliance. Circulation 110, 1799–1805 (2004).
Bernardo, B. C., Weeks, K. L., Pretorius, L. & McMullen, J. R. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol. Ther. 128, 191–227 (2010).
Chesky, J. A., LaFollette, S., Travis, M. & Fortado, C. Effect of physical training on myocardial enzyme activities in aging rats. J. Appl. Physiol. 55, 1349–1353 (1983).
Short, K. R. et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl Acad. Sci. USA 102, 5618–5623 (2005).
Ames, B. N., Shigenaga, M. K. & Hagen, T. M. Mitochondrial decay in aging. Biochim. Biophys. Acta 1271, 165–170 (1995).
Escobales, N. et al. Mitochondria-targeted ROS scavenger improves post-ischemic recovery of cardiac function and attenuates mitochondrial abnormalities in aged rats. J. Mol. Cell. Cardiol. 77, 136–146 (2014).
Hosseini, L., Vafaee, M. S. & Badalzadeh, R. Melatonin and nicotinamide mononucleotide attenuate myocardial ischemia/reperfusion injury via modulation of mitochondrial function and hemodynamic parameters in aged rats. J. Cardiovasc. Pharmacol. Ther. 25, 240–250 (2020).
Boengler, K., Kosiol, M., Mayr, M., Schulz, R. & Rohrbach, S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopenia Muscle 8, 349–369 (2017).
Judge, S. et al. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1564–R1572 (2005).
Picard, M. et al. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J. Appl. Phyisiol. 115, 1562–1571 (2013).
Wang, H. et al. Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1α and Akt activation. Cell Physiol. Biochem. 35, 2159–2168 (2015).
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009).
Laugwitz, K. L. et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433, 647–653 (2005).
Lázár, E., Sadek, H. A. & Bergmann, O. Cardiomyocyte renewal in the human heart: insights from the fall-out. Eur. Heart J. 38, 2333–2342 (2017).
Vujic, A. et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat. Commun. 9, 1659 (2018).
Mazzeo, R. S. & Tanaka, H. Exercise prescription for the elderly: current recommendations. Sports Med. 31, 809–818 (2001).
Singh, M. A. Exercise comes of age: rationale and recommendations for a geriatric exercise prescription. J. Gerontol. A Biol. Sci. Med. Sci. 57, M262–M282 (2002).
Gomes Neto, M. et al. High intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure with reduced ejection fraction: a systematic review and meta-analysis. Int. J. Cardiol. 261, 134–141 (2018).
Rognmo, Ø. et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 126, 1436–1440 (2012).
Chien, K. R., Knowlton, K. U., Zhu, H. & Chien, S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 5, 3037–3046 (1991).
Strøm, C. C. et al. Expression profiling reveals differences in metabolic gene expression between exercise-induced cardiac effects and maladaptive cardiac hypertrophy. FEBS J. 272, 2684–2695 (2005).
Song, H. K., Hong, S. E., Kim, T. & Kim, D. H. Deep RNA sequencing reveals novel cardiac transcriptomic signatures for physiological and pathological hypertrophy. PLoS ONE 7, e35552 (2012).
Bernardo, B. C., Ooi, J. Y. Y., Weeks, K. L., Patterson, N. L. & McMullen, J. R. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: current knowledge and emerging concepts. Physiol. Rev. 98, 419–475 (2018).
Alessio, H. M., Ansinelli, H., Threadgill, C. & Hagerman, A. E. Comparison of gene and protein expressions in rats residing in standard cages with those having access to an exercise wheel. BioMed. Res. Int. 2014, 950516 (2014).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Baar, K. et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 16, 1879–1886 (2002).
Botta, A. et al. Short term exercise induces PGC-1α, ameliorates inflammation and increases mitochondrial membrane proteins but fails to increase respiratory enzymes in aging diabetic hearts. PLoS ONE 8, e70248 (2013).
Arany, Z. et al. Transcriptional coactivator PGC-1α controls the energy state and contractile function of cardiac muscle. Cell Metab. 1, 259–271 (2005). The article shows how PGC-1α functions as a major regulator of bioenergetics in cardiac muscle.
Scarpulla, R. C., Vega, R. B. & Kelly, D. P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 23, 459–466 (2012).
Dufour, C. R. et al. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 5, 345–356 (2007).
Moreira, J. B. N. et al. Exercise reveals proline dehydrogenase as a potential target in heart failure. Prog. Cardiovasc. Dis. 62, 193–202 (2019).
Makarewich, C. A. et al. MOXI Is a mitochondrial micropeptide that enhances fatty acid β-oxidation. Cell Rep. 23, 3701–3709 (2018).
McMullen, J. R. et al. Phosphoinositide 3-kinase(p110α) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc. Natl Acad. Sci. USA 100, 12355–12360 (2003). This article shows that PI3K is required for the induction of physiological cardiac growth and is essential for maintaining contractile function in response to pathological stimuli.
McMullen, J. R. et al. Protective effects of exercise and phosphoinositide 3-kinase(p110α) signaling in dilated and hypertrophic cardiomyopathy. Proc. Natl Acad. Sci. USA 104, 612–617 (2007).
DeBosch, B. et al. Akt1 is required for physiological cardiac growth. Circulation 113, 2097–2104 (2006).
Kim, J. et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol. Endocrinol. 22, 2531–2543 (2008).
McMullen, J. R. et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110α) pathway. J. Biol. Chem. 279, 4782–4793 (2004).
Boudina, S. et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation 119, 1272–1283 (2009).
Noh, J. et al. Phosphoinositide dependent protein kinase 1 is required for exercise-induced cardiac hypertrophy but not the associated mitochondrial adaptations. J. Mol. Cell. Cardiol. 89, 297–305 (2015).
Kim, A. H., Khursigara, G., Sun, X., Franke, T. F. & Chao, M. V. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21, 893–901 (2001).
Weeks, K. L., Bernardo, B. C., Ooi, J. Y. Y., Patterson, N. L. & McMullen, J. R. The IGF1-PI3K-Akt signaling pathway in mediating exercise-induced cardiac hypertrophy and protection. Adv. Exp. Med. Biol. 1000, 187–210 (2017).
Vega, R. B., Konhilas, J. P., Kelly, D. P. & Leinwand, L. A. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab. 25, 1012–1026 (2017).
Silva, G. J. J., Bye, A., El Azzouzi, H. & Wisløff, U. MicroRNAs as important regulators of exercise adaptation. Prog. Cardiovasc. Dis. 60, 130–151 (2017).
Shi, J. et al. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics 7, 664–676 (2017).
Carè, A. et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 13, 613–618 (2007).
Liu, X. et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 21, 584–595 (2015).
Boström, P. et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143, 1072–1083 (2010).
Bezzerides, V. J. et al. CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight 1, e85904 (2016).
Hamilton, K. L. et al. Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic. Biol. Med. 34, 800–809 (2003).
Hutter, J. J. et al. Overexpression of heat shock protein 72 in transgenic mice decreases infarct size in vivo. Circulation 94, 1408–1411 (1996).
Tekin, D., Dursun, A. D. & Xi, L. Hypoxia inducible factor 1 (HIF-1) and cardioprotection. Acta Pharmacol. Sin. 31, 1085–1094 (2010).
Brown, D. A. et al. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J. Physiol. (Lond.) 569, 913–924 (2005).
Yao, Z. & Gross, G. J. Effects of the KATP channel opener bimakalim on coronary blood flow, monophasic action potential duration, and infarct size in dogs. Circulation 89, 1769–1775 (1994).
Wang, Z. et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J. Cardiovasc. Pharmacol. 72, 259–269 (2018).
Otaka, N. et al. Myonectin is an exercise-induced myokine that protects the heart from ischemia-reperfusion injury. Circ. Res. 123, 1326–1338 (2018).
Tham, Y. K. et al. Lipidomic profiles of the heart and circulation in response to exercise versus cardiac pathology: a resource of potential biomarkers and drug targets. Cell Rep. 24, 2757–2772 (2018).
Guo, H., Isserlin, R., Emili, A. & Burniston, J. G. Exercise-responsive phosphoproteins in the heart. J. Mol. Cell. Cardiol. 111, 61–68 (2017).
Penny, W. F. & Hammond, H. K. Randomized clinical trials of gene transfer for heart failure with reduced ejection fraction. Hum. Gene Ther. 28, 378–384 (2017).
Narkar, V. A. et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 134, 405–415 (2008).
Acknowledgements
J.B.N.M. is supported by a grant from the Research Council of Norway (project 275714). M.W. is supported by grants from the KG Jebsen Center for Exercise in Medicine and the Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU).
Author information
Authors and Affiliations
Contributions
Idea and outline: U.W. and J.B.N.M. Drafting of the manuscript: J.B.N.M., U.W. and M.W. Critical revision of the manuscript for intellectual content: all authors. Study supervision: U.W. Obtaining funding: U.W. and J.B.N.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Pooja Jha.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moreira, J.B.N., Wohlwend, M. & Wisløff, U. Exercise and cardiac health: physiological and molecular insights. Nat Metab 2, 829–839 (2020). https://doi.org/10.1038/s42255-020-0262-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0262-1
This article is cited by
-
Exercise training decreases lactylation and prevents myocardial ischemia–reperfusion injury by inhibiting YTHDF2
Basic Research in Cardiology (2024)
-
Effects of 12-week Tai Chi program on physical function, depression, and quality of life among cognitively impaired older adults: a feasibility study
BMC Geriatrics (2023)
-
Mitochondrial transplantation as a possible therapeutic option for sarcopenia
Journal of Molecular Medicine (2023)
-
Systolic and diastolic function during cycling at the respiratory threshold between elderly and young healthy individuals
Scientific Reports (2022)
-
METTL14 is required for exercise-induced cardiac hypertrophy and protects against myocardial ischemia-reperfusion injury
Nature Communications (2022)