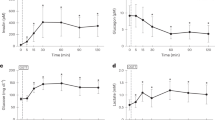
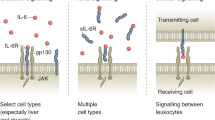
Abstract
Lactate, perhaps the best-known metabolic waste product, was first isolated from sour milk, in which it is produced by lactobacilli. Whereas microbes also generate other fermentation products, such as ethanol or acetone, lactate dominates in mammals. Lactate production increases when the demand for ATP and oxygen exceeds supply, as occurs during intense exercise and ischaemia. The build-up of lactate in stressed muscle and ischaemic tissues has established lactate’s reputation as a deleterious waste product. In this Perspective, we summarize emerging evidence that, in mammals, lactate also serves as a major circulating carbohydrate fuel. By providing mammalian cells with both a convenient source and sink for three-carbon compounds, circulating lactate enables the uncoupling of carbohydrate-driven mitochondrial energy generation from glycolysis. Lactate and pyruvate together serve as a circulating redox buffer that equilibrates the NADH/NAD ratio across cells and tissues. This reconceptualization of lactate as a fuel—analogous to how Hans Christian Andersen’s ugly duckling is actually a beautiful swan—has the potential to reshape the field of energy metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chen, Y.-J. et al. Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol. 12, 937–943 (2016).
Barron, J. T., Gu, L. & Parrillo, J. E. Malate-aspartate shuttle, cytoplasmic NADH redox potential, and energetics in vascular smooth muscle. J. Mol. Cell. Cardiol. 30, 1571–1579 (1998).
LaNoue, K. F. & Williamson, J. R. Interrelationships between malate-aspartate shuttle and citric acid cycle in rat heart mitochondria. Metabolism 20, 119–140 (1971).
Houstĕk, J., Cannon, B. & Lindberg, O. Gylcerol-3-phosphate shuttle and its function in intermediary metabolism of hamster brown-adipose tissue. Eur. J. Biochem. 54, 11–18 (1975).
Jorfeldt, L., Juhlin-Dannfelt, A. & Karlsson, J. Lactate release in relation to tissue lactate in human skeletal muscle during exercise. J. Appl. Physiol. 44, 350–352 (1978).
Warburg, O., Wind, F. & Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 8, 519–530 (1927).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Vyas, S., Zaganjor, E. & Haigis, M. C. Mitochondria and cancer. Cell 166, 555–566 (2016).
Wallace, D. C. Mitochondria and cancer. Nat. Rev. Cancer 12, 685–698 (2012).
Zong, W. X., Rabinowitz, J. D. & White, E. Mitochondria and cancer. Mol. Cell 61, 667–676 (2016).
Rathmell, J. C., Vander Heiden, M. G., Harris, M. H., Frauwirth, K. A. & Thompson, C. B. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell 6, 683–692 (2000).
Lemons, J. M. et al. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 8, e1000514 (2010).
Cantor, J. R. et al. Physiologic medium rewires cellular metabolism and reveals uric acid as an endogenous inhibitor of UMP synthase. Cell 169, 258–272.e17 (2017).
Vander Heiden, M. G. et al. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol. Cell. Biol. 21, 5899–5912 (2001).
Cori, C. F. Glycogen breakdown and synthesis in animal tissues. Endocrinology 26, 285–296 (1940).
Wahren, J., Felig, P., Ahlborg, G. & Jorfeldt, L. Glucose metabolism during leg exercise in man. J. Clin. Invest. 50, 2715–2725 (1971).
Jang, C. et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 30, 594–606.e3 (2019).
Dunn, A., Katz, J., Golden, S. & Chenoweth, M. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am. J. Physiol. 230, 1159–1162 (1976).
Katz, J., Okajima, F., Chenoweth, M. & Dunn, A. The determination of lactate turnover in vivo with 3H- and 14C-labelled lactate. The significance of sites of tracer administration and sampling. Biochem. J. 194, 513–524 (1981).
Stanley, W. C. et al. Lactate extraction during net lactate release in legs of humans during exercise. J. Appl. Physiol. 60, 1116–1120 (1986).
Wolfe, R. R. Isotopic measurement of glucose and lactate kinetics. Ann. Med. 22, 163–170 (1990).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371.e9 (2017).
Halestrap, A. P. The monocarboxylate transporter family: structure and functional characterization. IUBMB Life 64, 1–9 (2012).
Brooks, G. A. The science and translation of lactate shuttle theory. Cell Metab. 27, 757–785 (2018).
Zhao, C., Wilson, M. C., Schuit, F., Halestrap, A. P. & Rutter, G. A. Expression and distribution of lactate/monocarboxylate transporter isoforms in pancreatic islets and the exocrine pancreas. Diabetes 50, 361–366 (2001).
Sekine, N. et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells: potential role in nutrient sensing. J. Biol. Chem. 269, 4895–4902 (1994).
Wirthensohn, G. & Guder, W. G. Renal substrate metabolism. Physiol. Rev. 66, 469–497 (1986).
Mårin, P., Rebuffé-Scrive, M., Smith, U. & Björntorp, P. Glucose uptake in human adipose tissue. Metabolism 36, 1154–1160 (1987).
Gustafsson, J., Eriksson, J. & Marcus, C. Glucose metabolism in human adipose tissue studied by 13C-glucose and microdialysis. Scand. J. Clin. Lab. Invest. 67, 155–164 (2007).
DiGirolamo, M., Newby, F. D. & Lovejoy, J. Lactate production in adipose tissue: a regulated function with extra-adipose implications. FASEB J. 6, 2405–2412 (1992).
Tozzo, E., Shepherd, P. R., Gnudi, L. & Kahn, B. B. Transgenic GLUT-4 overexpression in fat enhances glucose metabolism: preferential effect on fatty acid synthesis. Am. J. Physiol. 268, E956–E964 (1995).
Jansson, P. A., Smith, U. & Lönnroth, P. Evidence for lactate production by human adipose tissue in vivo. Diabetologia 33, 253–256 (1990).
Boellaard, R. et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur. J. Nucl. Med. Mol. Imaging 42, 328–354 (2015).
Macintyre, A. N. et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72 (2014).
Pucino, V. et al. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4+ T cell metabolic rewiring. Cell Metab. 30, 1055–1074.e8 (2019).
Zhang, J. et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab. 31, 1136–1153.e7 (2020).
Angelin, A. et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293.e7 (2017).
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 24, 657–671 (2016).
Mountassif, D. et al. Immunoaffinity purification and characterization of glyceraldehyde-3-phosphate dehydrogenase from human erythrocytes. Acta Biochim. Biophys. Sin. (Shanghai) 41, 399–406 (2009).
Talaiezadeh, A., Shahriari, A., Tabandeh, M. R., Fathizadeh, P. & Mansouri, S. Kinetic characterization of lactate dehydrogenase in normal and malignant human breast tissues. Cancer Cell Int. 15, 19 (2015).
Mintun, M. A., Vlassenko, A. G., Rundle, M. M. & Raichle, M. E. Increased lactate/pyruvate ratio augments blood flow in physiologically activated human brain. Proc. Natl Acad. Sci. USA 101, 659–664 (2004).
Lambeth, M. J. & Kushmerick, M. J. A computational model for glycogenolysis in skeletal muscle. Ann. Biomed. Eng. 30, 808–827 (2002).
Williamson, D. H., Lund, P. & Krebs, H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem. J. 103, 514–527 (1967).
McClelland, G. B., Khanna, S., González, G. F., Butz, C. E. & Brooks, G. A. Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system? Biochem. Biophys. Res. Commun. 304, 130–135 (2003).
Patgiri, A. et al. An engineered enzyme that targets circulating lactate to alleviate intracellular NADH:NAD+ imbalance. Nat. Biotechnol. 38, 309–313 (2020).
Jitrapakdee, S., Vidal-Puig, A. & Wallace, J. C. Anaplerotic roles of pyruvate carboxylase in mammalian tissues. Cell. Mol. Life Sci. 63, 843–854 (2006).
Holness, M. J. & Sugden, M. C. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem. Soc. Trans. 31, 1143–1151 (2003).
Bowker-Kinley, M. M., Davis, W. I., Wu, P., Harris, R. A. & Popov, K. M. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem. J. 329, 191–196 (1998).
Sukonina, V. et al. FOXK1 and FOXK2 regulate aerobic glycolysis. Nature 566, 279–283 (2019).
Gopal, E. et al. Expression of slc5a8 in kidney and its role in Na+-coupled transport of lactate. J. Biol. Chem. 279, 44522–44532 (2004).
Scheiman, J. et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 25, 1104–1109 (2019).
Madaan, A. et al. Lactate produced during labor modulates uterine inflammation via GPR81 (HCA1). Am. J. Obstet. Gynecol. 216, 60.e1–60.e17 (2017).
Acknowledgements
S.E. is supported by the Swedish Research Council (2019-00773, 2018-02537), The Knut and Alice Wallenberg Foundation, Sahlgrenska’s University Hospital (LUA-ALF) and Novo Nordisk Foundation. J.D.R. is supported by NIH Pioneer award 1DP1DK113643, Diabetes Research Center grant P30 DK019525 and Pfizer, Inc.
Author information
Authors and Affiliations
Contributions
J.R. and S.E. equally conceived the idea, selected content and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.D.R. is a cofounder and stockholder in VL54 and Raze Therapeutics, and an advisor and stockholder in Agios Pharmaceuticals, Kadmon Pharmaceuticals, Bantam Pharmaceuticals, Colorado Research Partners, Rafael Pharmaceuticals and L.E.A.F. Pharmaceuticals. S.E. declares no competing interests.
Additional information
Peer review information Primary Handling Editor: Christoph Schmitt.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rabinowitz, J.D., Enerbäck, S. Lactate: the ugly duckling of energy metabolism. Nat Metab 2, 566–571 (2020). https://doi.org/10.1038/s42255-020-0243-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0243-4
This article is cited by
-
Lactate infusion elevates cardiac output through increased heart rate and decreased vascular resistance: a randomised, blinded, crossover trial in a healthy porcine model
Journal of Translational Medicine (2024)
-
Cancer metabolism and carcinogenesis
Experimental Hematology & Oncology (2024)
-
Comprehensive multiscale analysis of lactate metabolic dynamics in vitro and in vivo using highly responsive biosensors
Nature Protocols (2024)
-
Radiation-induced changes in energy metabolism result in mitochondrial dysfunction in salivary glands
Scientific Reports (2024)
-
TGF-β-driven LIF expression influences neutrophil extracellular traps (NETs) and contributes to peritoneal metastasis in gastric cancer
Cell Death & Disease (2024)