Abstract
Acute kidney injury (AKI) is strongly associated with mortality, independently of its cause. The kidney contributes to up to 40% of systemic glucose production by gluconeogenesis during fasting and under stress conditions. Whether kidney gluconeogenesis is impaired during AKI and how this might influence systemic metabolism remain unknown. Here we show that glucose production and lactate clearance are impaired during human and experimental AKI by using renal arteriovenous catheterization in patients, lactate tolerance testing in mice and glucose isotope labelling in rats. Single-cell transcriptomics reveal that gluconeogenesis is impaired in proximal tubule cells during AKI. In a retrospective cohort of critically ill patients, we demonstrate that altered glucose metabolism during AKI is a major determinant of systemic glucose and lactate levels and is strongly associated with mortality. Thiamine supplementation increases lactate clearance without modifying renal function in mice with AKI, enhances glucose production by renal tubular cells ex vivo and is associated with reduced mortality and improvement of the metabolic pattern in a retrospective cohort of critically ill patients with AKI. This study highlights an unappreciated systemic role of renal glucose and lactate metabolism under stress conditions, delineates general mechanisms of AKI-associated mortality and introduces a potential intervention targeting metabolism for a highly prevalent clinical condition with limited therapeutic options.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
RNA-seq data for human kidney biopsies are available at GEO (GSE126805). Mouse RNA-seq data are available at GEO (GSE52004) and ref. 50 and as a supplementary table in ref. 18. snRNA-seq data are available at GEO (GSE151167). Clinical data are available from the corresponding authors upon reasonable request. Raw data are included in this published article (and its Supplementary Information files). Source data are provided with this paper.
Code availability
Custom code will be made available on gitlab.unige.ch following publication.
Change history
17 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s42255-020-00283-6
References
Hoste, E. A. J. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423 (2015).
Legouis, D. et al. Rapid occurrence of chronic kidney disease in patients experiencing reversible acute kidney injury after cardiac surgery. Anesthesiology 126, 39–46 (2017).
Gaudry, S. et al. Initiation strategies for renal replacement therapy in the intensive care unit. N. Engl. J. Med. 375, 122–133 (2016).
Barbar, S. D. et al. Timing of renal replacement therapy in patients with acute kidney injury and sepsis. N. Engl. J. Med. 379, 1431–1442 (2018).
Li, P. et al. High-dose versus low-dose haemofiltration for the treatment of critically ill patients with acute kidney injury: an updated systematic review and meta-analysis. BMJ Open 7, e014171 (2017).
Billings, F. T. et al. High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA 315, 877–888 (2016).
Meybohm, P. et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N. Engl. J. Med. 373, 1397–1407 (2015).
Gerich, J. E., Meyer, C., Woerle, H. J. & Stumvoll, M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24, 382–391 (2001).
Epstein, F. H. Oxygen and renal metabolism. Kidney Int. 51, 381–385 (1997).
Meyer, C., Dostou, J. M. & Gerich, J. E. Role of the human kidney in glucose counterregulation. Diabetes 48, 943–948 (1999).
Yudkin, J. & Cohen, R. D. The contribution of the kidney to the removal of a lactic acid load under normal and acidotic conditions in the conscious rat. Clin. Sci. Mol. Med. 48, 121–131 (1975).
Sa, H. et al. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med. 42, 202–210 (2016).
Cippà, P. E. et al. Transcriptional trajectories of human kidney injury progression. JCI Insight 3, e123151 (2018).
Cippà, P. E. et al. A late B lymphocyte action in dysfunctional tissue repair following kidney injury and transplantation. Nat. Commun. 10, 1157 (2019).
Chen, Z. et al. Hepatocyte TRAF3 promotes insulin resistance and type 2 diabetes in mice with obesity. Mol. Metab. 4, 951–960 (2015).
Tigas, S. K., Sunehag, A. L. & Haymond, M. W. Impact of duration of infusion and choice of isotope label on isotope recycling in glucose homeostasis. Diabetes 51, 3170–3175 (2002).
Wu, H., Kirita, Y., Donnelly, E. L. & Humphreys, B. D. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J. Am. Soc. Nephrol. 30, 23–32 (2019).
Liu, J. et al. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight 2, e94716 (2017).
Ayyaz, A. et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569, 121–125 (2019).
Lee, J. W., Chou, C.-L. & Knepper, M. A. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J. Am. Soc. Nephrol. 26, 2669–2677 (2015).
Depeint, F., Bruce, W. R., Shangari, N., Mehta, R. & O’Brien, P. J. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 163, 94–112 (2006).
Paquet, R. J. & Mehlman, M. A. Thiamin deficiency. Effect of fatty acids on glucose synthesis in kidney cortex slices from thiamin-deficient rats. J. Biol. Chem. 247, 4905–4907 (1972).
Paquet, R. J., Mackerer, C. R. & Mehlman, M. A. Effects of thiamine deficiency and octanoate administration, in vivo, on gluconeogenesis in rat kidney slices and on amino acid profile in rat liver. Proc. Soc. Exp. Biol. Med. 140, 934–936 (1972).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 1–11 (2014).
Poyan Mehr, A. et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat. Med. 24, 1351–1359 (2018).
Tran, M. T. et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531, 528–532 (2016).
Lemos, D. R. et al. Interleukin-1β Activates a MYC-dependent metabolic switch in kidney stromal cells necessary for progressive tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 29, 1690–1705 (2018).
Zager, R. A., Johnson, A. C. M. & Becker, K. Renal cortical pyruvate depletion during AKI. J. Am. Soc. Nephrol. 25, 998–1012 (2014).
Hakimi, P. et al. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr. Metab. 2, 33 (2005).
Burgess, S. C. et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 279, 48941–48949 (2004).
Levraut, J. et al. Low exogenous lactate clearance as an early predictor of mortality in normolactatemic critically ill septic patients. Crit. Care Med. 31, 705–710 (2003).
Freire Jorge, P. et al. The association of early combined lactate and glucose levels with subsequent renal and liver dysfunction and hospital mortality in critically ill patients. Crit. Care 21, 218 (2017).
Fischer, K. F., Lees, J. A. & Newman, J. H. Hypoglycemia in hospitalized patients. Causes and outcomes. N. Engl. J. Med. 315, 1245–1250 (1986).
Haviv, Y. S., Sharkia, M. & Safadi, R. Hypoglycemia in patients with renal failure. Ren. Fail. 22, 219–223 (2000).
Moskowitz, A. et al. Thiamine as a renal protective agent in septic shock. a secondary analysis of a randomized, double-blind, placebo-controlled trial. Ann. Am. Thorac. Soc. 14, 737–741 (2017).
Lan, R. et al. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J. Am. Soc. Nephrol. 27, 3356–3367 (2016).
Katsyuba, E. et al. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature 563, 354–359 (2018).
Ricksten, S.-E., Bragadottir, G. & Redfors, B. Renal oxygenation in clinical acute kidney injury. Crit. Care 17, 221 (2013).
Singh, P., Ricksten, S.-E., Bragadottir, G., Redfors, B. & Nordquist, L. Renal oxygenation and haemodynamics in acute kidney injury and chronic kidney disease. Clin. Exp. Pharmacol. Physiol. 40, 138–147 (2013).
Redfors, B., Bragadottir, G., Sellgren, J., Swärd, K. & Ricksten, S.-E. Acute renal failure is NOT an “acute renal success”—a clinical study on the renal oxygen supply/demand relationship in acute kidney injury. Crit. Care Med. 38, 1695–1701 (2010).
Kellum, J. A. & Lameire, N., KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit. Care 17, 204 (2013).
Chung, S. T., Chacko, S. K., Sunehag, A. L. & Haymond, M. W. Measurements of gluconeogenesis and glycogenolysis: a methodological review. Diabetes 64, 3996–4010 (2015).
Allsop, J. R., Wolfe, R. R. & Burke, J. F. Tracer priming the bicarbonate pool. J. Appl. Physiol. 45, 137–139 (1978).
McCabe, B. J. & Previs, S. F. Using isotope tracers to study metabolism: application in mouse models. Metab. Eng. 6, 25–35 (2004).
Ekberg, K. et al. Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes 48, 292–298 (1999).
Kobayashi, A. et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169–181 (2008).
Wu, Y., Tamayo, P. & Zhang, K. Visualizing and interpreting single-cell gene expression datasets with similarity weighted nonnegative embedding. Cell Syst. 7, 656–666 (2018).
Schubert, M. et al. Perturbation-response genes reveal signaling footprints in cancer gene expression. Nat. Commun. 9, 20 (2018).
Legouis, David et al. Ex vivo analysis of renal proximal tubular cells. BMC Cell Biol. 16, 12 (2015).
Liu, J. et al. Cell-specific translational profiling in acute kidney injury. J. Clin. Invest. 124, 1242–1254 (2014).
Acknowledgements
This study was funded by the Swiss National Science Foundation (grant nos. PP00P3 157454 to S.S. and 167773 to P.E.C.), by a STARTER grant (no. RS03-25) to S.S. and D.L. from the HUG private foundation (the foundation of Geneva University Hospitals and the University of Geneva’s Faculty of Medicine) and by The Eli and Edythe Broad Foundation (to P.C. and A.P.M.). P.G. was supported by the ATIP Avenir programme. We thank G. Alvarado, A. Ransick and A. Kim for technical support.
Author information
Authors and Affiliations
Contributions
D.L. and S.S. conceived and designed the project. S.-E.R. performed human renal catheterization. D.L., A.F., S.P., C.V., K.G., L.B., M.F., T.V. and K.K. performed experiments. D.L., P.-Y.M. and J.P. generated and analysed ICU datasets. P.E.C. and A.P.M conducted snRNA-seq experiments and analysis. M.N. and P.E.C. provided RNA-seq data from kidney allograft recipients and D.L. performed analyses. S.S., D.L. and P.E.C. performed data interpretation. P.G., A.H. and E.F. validated data interpretation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Pooja Jha.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
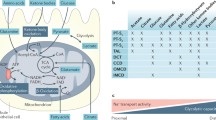
Extended Data Fig. 1 Kidney gluconeogenesis is downregulated in AKI.
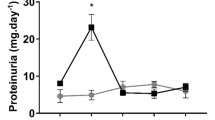
a, Unprocessed gels of representative western blot analysis for FBP1, PCK1, HK1 and PKM. Experiment was repeated twice with similar results b, Coomassie staining of the representative western blots. c, Liver mRNA expression of genes involved in gluconeogenesis (Fbp1, Pck1) and glycolysis (Hk1, Pkm) in sham versus IRI mice at 48 hours of reperfusion (IRI n=9 mice, sham n=8 mice). Data were shown as boxplots (median, interquartile, 2 and 95th percentiles) and compared with Wilcoxon two tailed test.
Extended Data Fig. 2 Validation of the snRNAseq analysis in the IRI model.
(a-c): Validation of the Neph-GFP mouse. Immunofluorescence from a representative example of a Neph-GFP mouse kidney showing GFP+ nuclei in Ltl+ PT cells and Umod+ TAL cells, but GFP- nuclei in Aqp2+ collecting duct cells. One representative example of >3 similar experiments is shown (d-j). A mouse model of moderate IRI to study tubular repair. d, Serum creatinine values measured 48h after IRI, with different ischemia times, as indicated. n=3 in sham and IRI 15 min, n=7 in IRI 10 min. Barplots represent mean and errorbars standard error of the mean. e, BUN values measure 48h and 28 days after IRI (15 minutes ischemia). N=5-12 mice, n=6 in sham, n= 12 in 48h, n=5 in 28d. Barplots represent mean and errorbars standard error of the mean. f, qPCR on renal tissue isolated at different time points after IRI with different ischemia times, as indicated. N= 6 in sham, n= 10 in IRI 10 min 48h, n= 6 in IRI 15 min 48h and n= 6 in IRI 15 min 28d. Data were compared using two tailed Wilcoxon test. Barplots represent mean and errorbars standard error of the mean. (g-j) One representative examples of >3 similar experiments is shown. Conventional H&E staining (g,i) and immunofluorescence (h,j) on kidney sections obtained 2 and 28 days after 15 min IRI showing the presence of damaged tubular cell at the cortico-medullary junction (S3 region of the PT). 28 days after IRI most tubular cells recovered, but still retained EDU (injected 48h after IRI), but some flattened cells and damaged tubules persisted (f).
Extended Data Fig. 3 snRNAseq analysis after IRI.
a, TSNE analysis on merged datasets including GFP+ nuclei at different time points after IRI as indicated. The line delineates proximal tubule (PT) cells. S1 and S3 indicate the corresponding segments of the PT. n=11,274 single cell transcriptomes from GFP+ cells obtained at different time points after injury (merged datasets); one representative example of 3 similar experiments is shown. b, Feature plots of representative genes, typically expressed by proximal tubule cells (Slc34a1), S1 cells (Slc5a12), S3 cells (Cyp7b1) and injured proximal tubule cells (Fth1). c, SWNE plot including PT nuclei isolated at different time points after IRI as presented in Fig. 2. The colors indicate the expression level of representative genes as indicated.
Extended Data Fig. 4 Upstream regulation of gluconeogenesis.
a, TMM normalized cpm of HNFA4 and PPARA genes in allograft kidney biopsies obtained at the end of the transplantation from brain-stem death donor (IRI group, n=25 patients) and 3 or 12 months after transplantation in patients with recovery renal graft status (control group, n=17 patients). b, relation between HNFA4 and PPARA genes with gluconeogenesis genes FBP1, PCK1 PCK2 and PC. BMI, body mass index. c, SWNE plots including PT nuclei isolated at different time points after IRI and highlighting the separation of PT cells in the early phase after IRI from steady-state condition. Representative regulators genes expressed in normal PT cells are embedded. The colours of the dots corresponds to the time point of harvesting after IRI. Violin plot and boxplot (with mean, IQR, 1st and 95th percentiles) are shown. Differential expression analyses were performed using genewise negative binomial model with quasi-likelihood test. Relations between genes expression were modelised using linear model. d, Heatmap showing the expression level of the pathways involved in cellular stress allograft kidney biopsies obtained at the end of the transplantation from brain-stem death donor (red color) and 3 or 12 months after transplantation in patients with recovery renal graft status (blue color). Lighter blue represents a downregulation whereas darker blue represents an upregulation of the pathway. e, Circular representation of Pearson correlation coefficient between each pathway and FBP1 (top) and HAVCR (bottom) expression in kidney biopsy sampled after reperfusion (n=42 patients). Blue triangles represent a positive, whereas red triangles represent a negative correlation. Levels of significant corresponds to transparency level. Violin plot and boxplot (with mean, IQR, 1st and 95th percentiles) are shown. Differential expression analyses were performed using genewise negative binomial model with quasi-likelihood test. Relation between genes expression was modelised using linear model. P-values for Pearson correlations were calculated using Fisher’s asymptotic test.
Extended Data Fig. 5 Impaired gluconeogenesis is associated to long term outcomes.
a, FBP1, PCK1, PCK2, HK1, PFKP and PKM genes expression data from RNAseq performed 3 and 12 months after transplantation and classified as recovery (n=23 patients), early transition to CKD (n=22 patients) and CKD (n=27 patients) using machine learning computational approach13.Relation between glycolytic and gluconeogenic gene expression and one-year renal function in kidney allograft recipients. Differential expression analyses were performed using genewise negative binomial model with quasi likelihood test with Benjamini-Hochberg correction for multiple test. Data are represented as violin plots and boxplot (mean, interquartile, 1st and 95th percentiles). b, Relation between one-year eGFR and FBP1, PC, PKM and PFKP gene expression at 3 different time points (during the transplantation, 3 and 12 months after n=38 patients for each time) in kidney allograft recipients. Each dot represents the coefficient (±95% CI) of the robust linear model fitting the relation between gene expression levels and one-year eGFR. Positive value indicates a positive association. c, Relation between FPB1 (top panel), PKM (bottom panel) genes expression at 3 months after transplantation and one-year GFR estimated by the CKD-EPI equation (n=38 patients). The regression was fitted using a robust linear model and p-values were calculated with a robust F-test.
Extended Data Fig. 6 Metabolic pattern in ICU patients.
a, Urinary glucose levels during human AKI according to KDIGO stage represented as violin plots with errors bars representing the interquartile range. Number of patients in each condition are shown in the figure. Groups were compared using ANOVA. b, Metabolism patterns definition: Scatter plot showing all the glucose and lactate values recorded in the ICU datasets (n=661’51). Five metabolism status were defined : baseline (lactate levels below median and with glucose levels between the 25th and the 50th percentile) ; impaired metabolism (lactate levels above the median with glucose level below the 75th percentile) ; isolated low glucose level (lactate levels below median with glucose levels above the 75th percentile) ; isolated high glucose level (lactate levels below median with glucose levels below the 25th percentile) and stress response (lactate levels above median and glucose levels above the 75th percentile). c, Flow chart of propensity score matching for AKI. d, Balance of variables for AKI before and after matching: Relative standardized differences between AKI and non-AKI patients, before and after propensity score matching for each variable included in the propensity score. A relative standardised difference less than 10% was considered to support the assumption of balance between groups. e, ICU mortality according to KDIGO and impaired metabolism status: Cumulative incidence curves for ICU mortality in the cohort of ICU patients matched using a propensity score for AKI comparing 4 groups (with or without impaired metabolism pattern and with or without AKI) stratified for KDIGO. f, Relation between proportion of metabolism status classified as impaired metabolism, for each allograft kidney recipients during the ICU stay and the post-operative day 15 serum creatinine levels, assessed by robust linear model (n=275) and the p-values were calculated from a robust F-test.
Extended Data Fig. 7 Thiamine administration in ICU patients.
a, Flow chart of propensity score matching for thiamine supplementation. b, Balance of variables for thiamine supplementation before and after matching: Relative standardized differences between patients receiving or not thiamine supplementation, with or without AKI, before and after propensity score matching for each variable included in the propensity score. A relative standardized difference less than 10% was considered to support the assumption of balance between groups. c, Cumulative incidence curves for ICU mortality in the non-AKI patients matched according to their thiamine supplementation status (n=263 patients in thiamine and n=526 in the non-thiamine groups respectively). Comparisons were performed with multivariate conditional logistic regression stratified on matched patients and generalized linear mixed model. d, Stacked barplots showing metabolism patterns over time in ICU patients free of AKI and receiving (n=263 patients) or not (n=526 patients) thiamine supplementation according to a propensity score matching. Comparisons were performed with multivariate conditional logistic regression stratified on matched patients and generalised linear mixed model. P-values were calculated from two tailed Wald test.
Supplementary information
Supplementary Information
Supplementary Tables 1–3
Source data
Source Data Fig. 1
Data.
Source Data Fig. 2
Data.
Source Data Fig. 5
Data.
Source Data Extended Data Fig. 1
Raw image for PCK1 and FBP1 proteins. Raw image for HK1 and PKM proteins.
Rights and permissions
About this article
Cite this article
Legouis, D., Ricksten, SE., Faivre, A. et al. Altered proximal tubular cell glucose metabolism during acute kidney injury is associated with mortality. Nat Metab 2, 732–743 (2020). https://doi.org/10.1038/s42255-020-0238-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0238-1
This article is cited by
-
Urinary PKM2, a marker predicating acute kidney injury in patients with sepsis
International Urology and Nephrology (2024)
-
Long-term viable chimeric nephrons generated from progenitor cells are a reliable model in cisplatin-induced toxicity
Communications Biology (2023)
-
Inhibition of PFKP in renal tubular epithelial cell restrains TGF-β induced glycolysis and renal fibrosis
Cell Death & Disease (2023)
-
Prognostic implication of lactic dehydrogenase-to-albumin ratio in critically ill patients with acute kidney injury
Clinical and Experimental Nephrology (2023)
-
Transcriptional regulation of proximal tubular metabolism in acute kidney injury
Pediatric Nephrology (2023)