Abstract
Following activation, macrophages undergo extensive metabolic rewiring1,2. Production of itaconate through the inducible enzyme IRG1 is a key hallmark of this process3. Itaconate inhibits succinate dehydrogenase4,5, has electrophilic properties6 and is associated with a change in cytokine production4. Here, we compare the metabolic, electrophilic and immunologic profiles of macrophages treated with unmodified itaconate and a panel of commonly used itaconate derivatives to examine its role. Using wild-type and Irg1−/− macrophages, we show that neither dimethyl itaconate, 4-octyl itaconate nor 4-monoethyl itaconate are converted to intracellular itaconate, while exogenous itaconic acid readily enters macrophages. We find that only dimethyl itaconate and 4-octyl itaconate induce a strong electrophilic stress response, in contrast to itaconate and 4-monoethyl itaconate. This correlates with their immunosuppressive phenotype: dimethyl itaconate and 4-octyl itaconate inhibited IκBζ and pro-interleukin (IL)-1β induction, as well as IL-6, IL-10 and interferon-β secretion, in an NRF2-independent manner. In contrast, itaconate treatment suppressed IL-1β secretion but not pro-IL-1β levels and, surprisingly, strongly enhanced lipopolysaccharide-induced interferon-β secretion. Consistently, Irg1−/− macrophages produced lower levels of interferon and reduced transcriptional activation of this pathway. Our work establishes itaconate as an immunoregulatory, rather than strictly immunosuppressive, metabolite and highlights the importance of using unmodified itaconate in future studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw and processed RNA-seq data have been deposited in the Gene Expression Omnibus with accession no. GSE145950. Source Data for the graphical representations found in Figs. 1–4 and Extended Data Figs. 1, 2 and 4–7 are provided. All other data that support the findings of this study are available from the corresponding author upon request.
References
Everts, B.et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood, 1422–1432 (2012).
Jha, A. K.et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 419–430 (2015).
Michelucci, A. et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA 110, 7820–7825 (2013).
Lampropoulou, V. et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 24, 158–166 (2016).
Cordes, T. et al. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J. Biol. Chem. 291, 14724–14284 (2016).
Bambouskova, M. et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature 556, 501–504 (2018).
Mills, E. L. et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117 (2018).
MacKenzie, E. D. et al. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol. Cell. Biol. 27, 3282–3289 (2007).
ElAzzouny, M. et al. Dimethyl itaconate is not metabolized into itaconate intracellularly. J. Biol. Chem. 292, 4766–4769 (2017).
Daniels, B. P. et al. The nucleotide sensor ZBP1 and kinase RIPK3 induce the enzyme IRG1 to promote an antiviral metabolic state in neurons. Immunity 50, 64–76 (2019).
Shen, H. et al. The human knockout gene CLYBL connects itaconate to vitamin B12. Cell 171, 771–782 (2017).
O’Neill, L. A. J. & Artyomov, M. N. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat. Rev. Immunol. 19, 273–281 (2019).
Adler, J., Wang, S.-F. & Lardy, H. A. The metabolism of itaconic acid by liver mitochondria. J. Biol. Chem. 229, 865–879 (1957).
Valls-Lacalle, L. et al. Succinate dehydrogenase inhibition with malonate during reperfusion reduces infarct size by preventing mitochondrial permeability transition. Cardiovasc. Res. 109, 374–384 (2016).
Valls-Lacalle, L. et al. Selective inhibition of succinate dehydrogenase in reperfused myocardium with intracoronary malonate reduces infarct size. Sci. Rep. 8, 2442 (2018).
Valls-Lacalle, L. et al. Author correction: Selective inhibition of succinate dehydrogenase in reperfused myocardium with intracoronary malonate reduces infarct size. Sci. Rep. 9, 6395 (2019).
Ackermann, W. W. & Potter, V. R. Enzyme inhibition in relation to chemotherapy. Proc. Soc. Exp. Biol. Med. 72, 1–9 (1949).
Harder, B. J., Bettenbrock, K. & Klamt, S. Model-based metabolic engineering enables high yield itaconic acid production by Escherichia coli. Metab. Eng. 38, 29–37 (2016).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Lu, W. et al. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a standalone Orbitrap mass spectrometer. Anal. Chem. 82, 3212–3221 (2010).
Melamud, E., Vastag, L. & Rabinowitz, J. D. Metabolomic analysis and visualization engine for LC−MS data. Anal. Chem. 82, 9818–9826 (2010).
Vincent, E. E. et al. Mitochondrial phosphoenolpyruvate carboxykinase regulates metabolic adaptation and enables glucose-independent tumor growth. Mol. Cell 60, 195–207 (2015).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Daly, M. J. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Acknowledgements
This work was supported by R01-A1125618 from the National Institute of Allergy and Infectious Diseases (NIAID) granted to M.N.A.
Author information
Authors and Affiliations
Contributions
A.S., M.B. and M.N.A. conceived and designed the study and wrote the manuscript, with contributions from V.C. and K.M.S. A.S. and M.B. performed all cell culture, cytokine, quantitative PCR, cell viability and biochemistry analyses. P.S.A. performed RNA-seq data analysis. H.K., V.C., D.M.S., T.P.R. and K.M.S. designed and performed mass spectrometry metabolic measurements and analysis. D.D. and K.A. provided 13C5-itaconic acid.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: George Caputa.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 BMDM viability after treatment with itaconate or itaconate derivatives.
a, Viability over time (3h, 9h, 12h, 18h) in unstimulated BMDMs treated with the indicated compounds (n = 3 cultures except for the following: 3h & 12h 5 ITA, 3h & 12 10 ITA n = 5 cultures; 3h & 12h 7.5 ITA, 3h & 12h 5 SA, 3h & 12h 5 MA n = 2 cultures (statistics not shown for n < 3); 9h & 18 5 ITA, 9h & 18h 10 ITA n = 6 cultures). b, Viability in BMDMs pre-treated with the indicated compounds for 3h (left) or 12h (right) prior to 6h LPS stimulation (n = 3 cultures except for the following: 5 ITA, 10 ITA for both treatment timepoints n = 6 cultures). MA = malonic acid, SA = succinic acid. Treatment concentrations for all experiments listed in mM. All bar plots representative of mean + s.e.m., except where n < 3 only the mean is depicted. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. P values are calculated using one-way ANOVA. Exact P values are listed in Source Data.
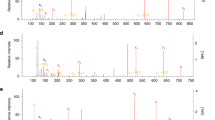
Extended Data Fig. 2 Dose dependent accumulation of itaconate and derivatives in macrophages.
a, b, Intracellular levels of 4OI (blue), 4EI (yellow), and itaconate (pink) after 3h (a) or 12h (b) treatment with the indicated compound in WT primary BMDMs (n = 4 cultures). Intracellular detection of DI is not amenable to the electrospray ionization LCMS method used for these experiments. c, Intracellular levels of itaconate in unstimulated and 6h LPS-stimulated WT BMDMs that were either untreated (Control, n = 3 cultures) or pre-treated for 3h prior to stimulation with 5 mM unlabeled (12C ITA-Sigma (commercially available itaconate), 12C ITA-CTRL (itaconate synthesized as a control for the labeled itaconate), n = 2 cultures) or 13C5-itaconate (13C ITA, n = 3 cultures). Exact distribution of data can be found in Supplementary Information in Source Data files. Treatment concentrations for all experiments listed in mM. All bar plots representative of mean + s.e.m., except where n < 3 only the mean is depicted. AU = arbitrary units based on mass spectrometry peak area, and is not directly comparable between experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. P values are calculated using one-way ANOVA, and exact P values are listed in Source Data.
Extended Data Fig. 3 13C Incorporation in unstimulated and LPS-activated macrophages after 13C Itaconate or 13C Succinate treatment.
Tables outlining the percentage of 13C incorporation into various metabolites after treatment with unlabeled and 13C-labeled itaconate (a, b) and succinate (c, d) in unstimulated BMDMs (a, c) and after 6h LPS treatment (b, d). 12C ITA-Sigma is commercially available itaconate, while 12C ITA-CTRL is a synthesized control itaconate prepared using similar methods to the preparation of 13C ITA. ND = Not Detected.
Extended Data Fig. 4 Neutral pH sodium itaconate enters cells and induces succinate accumulation.
a, The chemical structures of malonic acid, 2-methylsuccinic acid, and succinic acid. b, Intracellular levels of TCA cycle metabolites in unstimulated and 6h LPS-stimulated WT BMDMs that were either untreated (Control, n = 3 cultures) or pre-treated for 3h prior to stimulation with 5 mM unlabeled (12C SA, n = 2 cultures) or 13C4-succinate (13C SA, n = 3 cultures). Exact distribution of data can be found in Supplementary Information in Source Data files. c, d, Intracellular methylsuccinate (MSA) (c) and succinate (d) in unstimulated WT BMDMs after 3h treatment with 5 mM MSA (n = 3 cultures). e, f, Intracellular itaconate (left) and succinate (right) levels in unstimulated WT BMDMs after 3h (e) or 12h (f) treatment with 10 mM sodium itaconate (Na-ITA) or 5 mM itaconate (ITA) (n = 4 cultures). g, h, Viability after 3h (g) or 12h (h) treatment with 10 mM Na-ITA or 5 mM ITA followed by 24h LPS stimulation (n = 4 cultures). Treatment concentrations for all experiments listed in mM. All bar plots representative of mean + s.e.m., except where n < 3 only the mean is depicted. AU = arbitrary units based on mass spectrometry peak area and is not directly comparable between experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. P values are calculated using two-tailed, unpaired t test for c, d and one-way ANOVA for e, f, g, h. Exact P values are listed in Source Data.
Extended Data Fig. 5 Itaconate treatment increases succinate accumulation and inhibits SDH activity.
a, Intracellular itaconate versus intracellular succinate accumulation in BMDMs after 12h treatment with the indicated compounds (n = 8 cultures). b, Intracellular succinate levels in BMDMs after 12h treatment with DI (n = 8 cultures) c, SDH activity in the presence of the indicated compounds (n = 5 cultures except for 5 NaITA, 7.5 NaITA n = 3 cultures; 10 NaITA n = 2 cultures; statistics not shown for n < 3). All compounds were neutralized with sodium hydroxide before adding to the assay. *P < 0.05, ***P < 0.001, ****P < 0.0001, P values calculated using one way ANOVA. Exact P values listed in Supplementary Information in Source Data files. d, Time course of itaconate and succinate accumulation after LPS stimulation in WT BMDMs (n = 6 cultures). Treatment concentrations for all experiments listed in mM. All bar plots representative of mean + s.e.m., except where n < 3 only the mean is depicted. AU = arbitrary units based on mass spectrometry peak area and is not directly comparable between experiments.
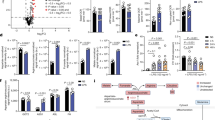
Extended Data Fig. 6 Cytokine levels in Nrf2-deficient macrophages.
a, Cytokine levels in WT BMDMs pre-treated with the indicated compounds for 3h followed by 4h LPS stimulation (for TNF n = 4 independent experiments, for IL-6 and IL-10 n = 3 independent experiments). b, Western blot of IκBζ expression in WT BMDMs pre-treated with the indicated compounds for 3h followed by 1h LPS stimulation. Westerns representative of 3 independent experiments. c, d, Cytokine levels in Nrf2−/− BMDMs pre-treated with the indicated compounds for 3h (c) or 12h (d) followed by 4h LPS stimulation, (n = 3 independent experiments) e, f, Western blot of IκBζ expression in Nrf2−/− BMDMs pre-treated with the indicated compounds for 3h (e) or 12h (f) followed by 1h LPS stimulation. Westerns representative of 3 independent experiments. Treatment concentrations for all experiments listed in mM. All bar plots representative of mean + s.e.m. For (a), (c), and (d) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, P values calculated by one way ANOVA. Exact P values listed in Supplementary Information in Source Data files.
Extended Data Fig. 7 Titrations demonstrate differences in cytokine regulation by itaconate and its derivatives.
a, b, c, d IL-6 (a), TNF (b), secreted IL-1β (c), and IFN-β (d) levels after treating WT BMDMs with the indicated compounds for 12h followed by 6h LPS (n = 3 independent experiments except for the following samples, which were n = 2 independent experiments. For IL-6: 2.5 SFN, 7.5 SFN; for TNF: 0.2 DI, 0.2 4OI, 2.5 SFN, 7.5 SFN; for IL-1β: 0.2 DI, 0.2 4OI, 2.5 SFN, 7.5 SFN; for IFN-β: Control, 0.2 DI, 7.5 SFN) Treatment concentrations for all experiments listed in mM. All bar plots representative of mean + s.e.m., except where n < 3 only the mean is depicted. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. P values are calculated using one-way ANOVA. Exact P values are listed in Source Data.
Supplementary information
Supplementary Information
Supplementary Table 1 and Fig. 1
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 2
Unprocessed immunoblots.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 3
Unprocessed immunoblots
Source Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 6
Unprocessed immunoblots.
Source Data Extended Data Fig. 7
Statistical Source Data.
Rights and permissions
About this article
Cite this article
Swain, A., Bambouskova, M., Kim , H. et al. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat Metab 2, 594–602 (2020). https://doi.org/10.1038/s42255-020-0210-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0210-0
This article is cited by
-
Anti-inflammatory and anti-oxidative electrospun nanofiber membrane promotes diabetic wound healing via macrophage modulation
Journal of Nanobiotechnology (2024)
-
Myeloid-derived suppressor cell mitochondrial fitness governs chemotherapeutic efficacy in hematologic malignancies
Nature Communications (2024)
-
Anti-Necroptotic Effects of Itaconate and its Derivatives
Inflammation (2024)
-
The Role of Pyroptosis and Autophagy in the Nervous System
Molecular Neurobiology (2024)
-
Metabolite itaconate in host immunoregulation and defense
Cellular & Molecular Biology Letters (2023)