Abstract
Plasticity of cancer metabolism can be a major obstacle to efficient targeting of tumour-specific metabolic vulnerabilities. Here, we identify the compensatory mechanisms following the inhibition of major pathways of central carbon metabolism in c-MYC-induced liver tumours. We find that, while inhibition of both glutaminase isoforms (Gls1 and Gls2) in tumours considerably delays tumourigenesis, glutamine catabolism continues, owing to the action of amidotransferases. Synergistic inhibition of both glutaminases and compensatory amidotransferases is required to block glutamine catabolism and proliferation of mouse and human tumour cells in vitro and in vivo. Gls1 deletion is also compensated for by glycolysis. Thus, co-inhibition of Gls1 and hexokinase 2 significantly affects Krebs cycle activity and tumour formation. Finally, the inhibition of biosynthesis of either serine (Psat1-KO) or fatty acid (Fasn-KO) is compensated for by uptake of circulating nutrients, and dietary restriction of both serine and glycine or fatty acids synergistically suppresses tumourigenesis. These results highlight the high flexibility of tumour metabolism and demonstrate that either pharmacological or dietary targeting of metabolic compensatory mechanisms can improve therapeutic outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All the data that support the findings of this study are available from the corresponding author upon request. The microarray data have been deposited in Gene Expression Omnibus (GEO) under accession code GSE129013. Source data for Figs. 1–7 and Extended Data Figs. 1 and 3–9 are presented with the paper.
References
Vander Heiden, M. G. & DeBerardinis, R. J. Understanding the intersections between metabolism and cancer biology. Cell 168, 657–669 (2017).
Hsu, P. P. & Sabatini, D. M. Cancer cell metabolism: Warburg and beyond. Cell 134, 703–707 (2008).
Luengo, A., Gui, D. Y. & Vander Heiden, M. G. Targeting metabolism for cancer therapy. Cell Chem. Biol. 24, 1161–1180 (2017).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of ras-driven non-small cell lung cancer. Cell Metab. 23, 517–528 (2016).
Lyssiotis, C. A. & Kimmelman, A. C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 27, 863–875 (2017).
Miller, D. M., Thomas, S. D., Islam, A., Muench, D. & Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 18, 5546–5553 (2012).
Stine, Z. E., Walton, Z. E., Altman, B. J., Hsieh, A. L. & Dang, C. V. MYC, Metabolism, and Cancer. Cancer Discov. 5, 1024–1039 (2015).
Dang, C. V., Reddy, E. P., Shokat, K. M. & Soucek, L. Drugging the ‘undruggable’ cancer targets. Nat. Rev. Cancer 17, 502–508 (2017).
Shachaf, C. M. et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature 431, 1112–1117 (2004).
Yuneva, M. O. et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 15, 157–170 (2012).
Hu, S. et al. 13C-pyruvate imaging reveals alterations in glycolysis that precede c-Myc-induced tumor formation and regression. Cell Metab. 14, 131–142 (2011).
Mendez-Lucas, A. et al. Glucose catabolism in liver tumors induced by c-MYC can be sustained by various PKM1/PKM2 ratios and pyruvate kinase activities. Cancer Res. 77, 4355–4364 (2017).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007).
Hensley, C. T., Wasti, A. T. & DeBerardinis, R. J. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Invest. 123, 3678–3684 (2013).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Still, E. R. & Yuneva, M. O. Hopefully devoted to Q: targeting glutamine addiction in cancer. Br. J. Cancer 116, 1375–1381 (2017).
Patra, K. C. et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 24, 213–228 (2013).
Xiang, Y. et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J .Clin. Invest. 125, 2293–2306 (2015).
DeWaal, D. et al. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 9, 446 (2018).
Svensson, R. U. et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat. Med. 22, 1108–1119 (2016).
Li, L. et al. Inactivation of fatty acid synthase impairs hepatocarcinogenesis driven by AKT in mice and humans. J. Hepatol. 64, 333–341 (2016).
Yang, C. et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol. Cell 56, 414–424 (2014).
Cheng, T. et al. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc. Natl Acad. Sci. USA 108, 8674–8679 (2011).
Momcilovic, M. et al. Targeted inhibition of EGFR and glutaminase induces metabolic crisis in EGFR mutant lung cancer. Cell Rep. 18, 601–610 (2017).
Momcilovic, M. et al. The GSK3 signaling axis regulates adaptive glutamine metabolism in lung squamous cell carcinoma. Cancer Cell 33, 905–921 e905 (2018).
Malato, Y. et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J. Clin. Invest. 121, 4850–4860 (2011).
Chen, X. & Calvisi, D. F. Hydrodynamic transfection for generation of novel mouse models for liver cancer research. Am. J. Pathol. 184, 912–923 (2014).
Zalkin, H. & Smith, J. L. Enzymes utilizing glutamine as an amide donor. Adv. Enzymol. Relat. Areas Mol. Biol. 72, 87–144 (1998).
Spinelli, J. B. et al. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science 358, 941–946 (2017).
Xiang, L. et al. Glutaminase 1 expression in colorectal cancer cells is induced by hypoxia and required for tumor growth, invasion, and metastatic colonization. Cell Death Dis. 10, 40 (2019).
Satoh, K. et al. Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by MYC. Proc. Natl Acad. Sci. USA 114, E7697–E7706 (2017).
Nagle, P. W., Plukker, J. T. M., Muijs, C. T., van Luijk, P. & Coppes, R. P. Patient-derived tumor organoids for prediction of cancer treatment response. Semin. Cancer Biol. 53, 258–264 (2018).
Finlay, M. R. V. et al. Discovery of a thiadiazole-pyridazine-based allosteric glutaminase 1 inhibitor series that demonstrates oral bioavailability and activity in tumor xenograft models. J. Med. Chem. 62, 6540–6560 (2019).
Alwarawrah, Y. et al. Fasnall, a selective FASN inhibitor, shows potent anti-tumor activity in the MMTV-Neu model of HER2(+) breast cancer. Cell Chem. Biol. 23, 678–688 (2016).
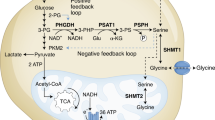
Mattaini, K. R., Sullivan, M. R. & Vander Heiden, M. G. The importance of serine metabolism in cancer. J. Cell Biol. 214, 249–257 (2016).
Pacold, M. E. et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 12, 452–458 (2016).
Labuschagne, C. F., van den Broek, N. J., Mackay, G. M., Vousden, K. H. & Maddocks, O. D. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 7, 1248–1258 (2014).
Gao, X. et al. Serine availability influences mitochondrial dynamics and function through lipid metabolism. Cell Rep. 22, 3507–3520 (2018).
Maddocks, O. D. K. et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544, 372–376 (2017).
Diehl, F. F., Lewis, C. A., Fiske, B. P. & Vander Heiden, M. G. Cellular redox state constrains serine synthesis and nucleotide production to impact cell proliferation. Nat. Metab. 1, 861–867 (2019).
Esaki, K. et al. L-Serine deficiency elicits intracellular accumulation of cytotoxic deoxysphingolipids and lipid body formation. J. Biol. Chem. 290, 14595–14609 (2015).
Li, L. et al. Differential requirement for de novo lipogenesis in cholangiocarcinoma and hepatocellular carcinoma of mice and humans. Hepatology 63, 1900–1913 (2016).
Thomas, A. G. et al. Kinetic characterization of ebselen, chelerythrine and apomorphine as glutaminase inhibitors. Biochem. Biophys. Res. Commun. 438, 243–248 (2013).
Thangavelu, K., Chong, Q. Y., Low, B. C. & Sivaraman, J. Structural basis for the active site inhibition mechanism of human kidney-type glutaminase (KGA). Sci. Rep. 4, 3827 (2014).
Rais, R. et al. Discovery of 6-diazo-5-oxo-l-norleucine (DON) prodrugs with enhanced CSF delivery in monkeys: a potential treatment for glioblastoma. J. Med. Chem. 59, 8621–8633 (2016).
Tenora, L. et al. Tumor-targeted delivery of 6-diazo-5-oxo-l-norleucine (DON) using substituted acetylated lysine prodrugs. J. Med. Chem. 62, 3524–3538 (2019).
Gravel, S. P. et al. Serine deprivation enhances antineoplastic activity of biguanides. Cancer Res. 74, 7521–7533 (2014).
Mullarky, E. et al. Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc. Natl Acad. Sci. USA 113, 1778–1783 (2016).
Sullivan, M. R. et al. Increased serine synthesis provides an advantage for tumors arising in tissues where serine levels are limiting. Cell Metab. 29, 1410–1421 e1414 (2019).
Patel, M. et al. Report of a first-in-human study of the first-in-class fatty acid synthase (FASN) inhibitor TVB-2640. Cancer Res. https://doi.org/10.1158/1538-7445.AM2015-CT203 (2015).
Pascual, G. et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45 (2017).
Chlebowski, R. T. et al. Association of low-fat dietary pattern with breast cancer overall survival: a secondary analysis of the women’s health initiative randomized clinical trial. JAMA Oncol. 4, e181212 (2018).
Mingote, S. et al. Genetic pharmacotherapy as an early CNS drug development strategy: testing glutaminase inhibition for schizophrenia treatment in adult mice. Front. Syst. Neurosci. 9, 165 (2015).
Schuler, M., Dierich, A., Chambon, P. & Metzger, D. Efficient temporally controlled targeted somatic mutagenesis in hepatocytes of the mouse. Genesis 39, 167–172 (2004).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Chow, E. K., Fan, L. L., Chen, X. & Bishop, J. M. Oncogene-specific formation of chemoresistant murine hepatic cancer stem cells. Hepatology 56, 1331–1341 (2012).
Tao, J. et al. Distinct anti-oncogenic effect of various microRNAs in different mouse models of liver cancer. Oncotarget 6, 6977–6988 (2015).
Li, Y., Choi, P. S., Casey, S. C. & Felsher, D. W. Activation of Cre recombinase alone can induce complete tumor regression. PLoS One 9, e107589 (2014).
Chakravarthy, M. V. et al. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 1, 309–322 (2005).
Marin-Valencia, I. et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 15, 827–837 (2012).
MacRae, J. I. et al. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 11, 67 (2013).
Zamboni, N., Fendt, S. M., Ruhl, M. & Sauer, U. (13)C-based metabolic flux analysis. Nat. Protoc. 4, 878–892 (2009).
Lewis, I. A., Schommer, S. C. & Markley, J. L. rNMR: open source software for identifying and quantifying metabolites in NMR spectra. Magn. Reson. Chem. 47 Suppl 1, S123–S126 (2009).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Miyoshi, H. & Stappenbeck, T. S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 8, 2471–2482 (2013).
Purwaha, P., Silva, L. P., Hawke, D. H., Weinstein, J. N. & Lorenzi, P. L. An artifact in LC-MS/MS measurement of glutamine and glutamic acid: in-source cyclization to pyroglutamic acid. Anal. Chem. 86, 5633–5637 (2014).
Sellers, K. et al. Metabolic reprogramming and Notch activity distinguish between non-small cell lung cancer subtypes. Br. J. Cancer 121, 51–64 (2019).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 6, pl1 (2013).
Acknowledgements
This work was supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001223), the UK Medical Research Council (FC001223) and the Wellcome Trust (FC001223); by grants from NIH R01CA136606, R21CA198490 and P30DK026743 for UCSF Liver Center to X.C.; NIH R01CA206167 and Department of Veteran Affairs BX000733 for N.H. We thank The Francis Crick Institute Biological Services for breeding and maintenance of the mice and the MRC Biomedical NMR Centre for assistance. We also thank A. P. Gould, P. M. Nunes and A. P. Bailey for critical reading and useful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
A.M.L. and M.Y. conceived the research project, designed and performed experiments and wrote the paper. W.L, P.C.D., N.L., J.I.M and L.N. performed research and/or contributed to analysis and discussion. S.R. and N.H. provided mouse models. V.L. and M.R.J. provided human and mouse organoids. M.C., Z.W and N.P.J. provided compound 27. X.C. and C.X. performed tissue microarray analysis and provided reagents.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary handling editor: George Caputa.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Glucose and glutamine metabolism in MYC liver tumours.
a–g, Mice bearing MYC-driven liver tumours and control mice (n=5 per group) were infused with [U-13C]glucose (a–c), or [U-13C]glutamine (d–f) and the label incorporation into tissue metabolites was analysed by GC-MS: percent enrichment in either serum glucose (a) or glutamine (d) in mice administered either [U-13C]glucose or [U-13C]glutamine bolus, respectively (*glutamine enrichment is estimated from quantification of its spontaneous product pyroglutamate); (b and e) percent enrichment; (c and g) total content of metabolites. Note that lower glutamine enrichment in Krebs cycle intermediates in tumours in comparison with normal livers is proportional to the difference in serum enrichment between control and tumour-bearing mice; normalized values for Krebs cycle metabolites from (b and e) are shown in (g). Data are presented as mean ± S.D. Statistical analysis was performed using a two-tailed Student’s t-test. Complete list of exact p-values is provided as a source data file. h, 15N-HMBC 2D NMR signals of the indicated metabolites in the indicated mouse tissues after amino-15N-glutamine bolus. Spectra of three representative mice per group are shown (n=5 mice per group). (i) Percent enrichment of tissue total fatty acids (both free and esterified) after either [U-13C]glucose or [U-13C]glutamine infusions (n=5 mice per group). GC-MS. Data are presented as mean ± S.D. Statistical analysis was performed using a two-tailed Student’s t-test. Complete list of exact P values is provided as a source data file. j, Nile red fluorescence visualised by confocal microscopy showing neutral lipid accumulation (in red) in MYC-driven tumours. Nuclei are shown in blue. Representative images out of four normal livers and tumour-bearing livers are shown.
Extended Data Fig. 2 Expression of key metabolic enzymes in MYC liver tumours and the method of genetically manipulating their expression in vivo.
a, A diagram depicting some of the most relevant transcriptomic changes in central carbon metabolism in MYC-driven liver tumours when compared with normal livers based on microarray data shown in (b). b, A heat map of gene expression of relevant metabolic enzymes and metabolite transporters in normal livers and MYC-driven liver tumours (n = 4 mice per group). Statistical analysis was performed using a two-tailed Student’s t-test. *, P < 0.05. c, Generation of liver specific conditional knockouts of the genes of interest in hepatocytes. CreERT2 is expressed under the hepatocyte-specific albumin gene promoter. Cre is activated upon administration of tamoxifen. Simultaneously, the Enhanced Yellow Fluorescent Protein (eYFP) reporter gene is activated, following Cre-mediated excision of a loxP-flanked transcriptional “stop” sequence, after the Rosa26 locus. d–g, To confirm a cell of origin of MYC-driven liver tumours, the dose of AAV8-Cre (adenoassociated virus serotype 8 expressing Cre recombinase) required to activate the eYFP reporter expression in all the hepatocytes was titrated. Dose is expressed in genome copies (GC): d, Macroscopic fluorescent image of the livers of the mice treated with different doses of AAV8-Cre; e, Confocal fluorescent image of the mouse livers shown in (d) demonstrating that the dose of 8 × 1012 GC induces eYFP expression in all the hepatocytes; f, Immunostaining with the cholangiocyte marker Pan-cytokeratin (PanCK) demonstrates that cholangiocytes are not targeted by AAV8-Cre; g, Rosa26-eYFP mice treated with 8 × 1012 GC AAV8-Cre, were hydrodynamically transfected with MYC and MCL1, and the resulting tumours expressed eYFP, demonstrating that hepatocytes are the cell of origin of the tumours (n = 3 mice per dose). h, MYC and MCL1 are among genes frequently upregulated in human liver cancers. Analysis performed in cBioPortal68,69, combining all available liver cancer databases, expressed as percentage of positive tumour samples.
Extended Data Fig. 3 Metabolic consequences of the deletion of either Gls1 or Gls2 in MYC liver tumours.
a, Total concentration of 13C-labelled Krebs cycle metabolites in CT and Gls1KO tumours after a [U-13C]glutamine bolus (n = 5 mice per group). GC-MS. b, Western blot of the samples presented in Fig. 2a showing the expression of GLS2 in Gls1KO tumours (normal livers n = 3 mice, CT tumours n = 5 mice, Gsl1KO tumours n = 5 mice). c–e, shRNA mediated Gls2 knock down in MYC-driven liver tumours with intact Gls1 expression does not affect tumour burden or glutamine catabolism. Liver tumours were induced by hydrodynamics-driven co-transfection of plasmids encoding MYC that included a miR30 based shRNA targeting for Gls2 or Renilla Luciferase (pT3-EF1α-c-MYC/shGls2 and pT3-EF1α-c-MYC/shLuc, respectively), and a plasmid encoding MCL1 (pT3-EF1α-MCL1) (c) Western blot demonstrating efficient Gls2 knock down (normal livers n = 3 mice, shLuc tumours n = 5 mice, shGls2 tumours n = 5 mice). d, Kaplan-Meier survival curve (shLuc n=13 mice; shGls2 n=11 mice). P value was calculated by Mantel–Cox test. e, 13C-enrichment in the indicated metabolites extracted from either shLuc or shGls2 tumours (Gls1 wild type) after a [U-13C]glutamine bolus (n = 5 mice per group). f, Total level of glutamine and glutamate in CT/shLuc (n = 6), Gls1KO/shLuc (n = 5), and Gls1KO/shGls2 (n = 6) tumours. LC–MS. g, Isotopologue distribution of the 13C-enrichment of glutamine in the serum of mice shown in Fig. 2h-j, Extended Data Fig. 3f,h and 4b (CT/shLuc n = 6 mice, Gls1KO/shLuc n = 4 mice, Gls1KO/Gls2KO n = 5 mice). GC-MS. Glutamine enrichment was estimated from quantification of its spontaneous product pyroglutamate. h, 13C-enrichment of the indicated metabolites after a [U-13C]glutamine bolus, related to Fig. 2h,i, shows the enrichment of glycolytic intermediates from [U-13C]glutamine through gluconeogenesis in tumours and the respective adjacent livers (CT/shLuc n = 6 mice, Gls1KO/shLuc n = 5 mice, Gls1KO/Gls2KO n = 6 mice). All data are presented as mean ± S.D. Statistical analysis was performed using a two-tailed Student’s t-test. Complete list of exact P values is provided as a source data file.
Extended Data Fig. 4 Non-essential amino acids support proliferation of tumour cells in the absence of glutaminases.
a, b, Total levels of NEAA measured in extracts from either CT and Gls1KO tumours (a; n=5 mice per group; GC-MS), or CT/shLuc and Gls1KO/shGls2 tumours (b; n=6 mice per group; GC-MS). c, Proliferation of cells derived from either CT, Gls1KO/shLuc or Gls1KO/shGls2 HCCMYC tumours in the indicated conditions. Representative curves from one of three independent experiments (with three biological replicates) are shown. d, Quantification of Krebs cycle metabolites and amino acids in extracts from the CT, Gls1KO/shLuc or Gls1KO/shGls2 HCCMYC cells grown in control conditions (Data represents the average of three different experiments). e, 15N-enrichment in the indicated amino acids from CT and Gls1KO/shGls2 tumours after a [15N]alanine bolus (n=4 mice per group). GC-MS. Data are presented as mean ± S.D. Statistical analysis was performed using a two-tailed Student’s t-test. Complete list of exact P values is provided as a source data file.
Extended Data Fig. 5 The role of transamidase-dependent glutamine catabolism.
a, A correlation between the gene expression level of Gls1 and different amidotransferases analyzed from the TCGA human Hepatocellular Carcinoma Provisional mRNA dataset (https://www.cancer.gov/tcga, RNA Seq V2, 371 patients / 373 samples). Modified from cBioPortal68,69. b, A representative full 1H-15N 2D-HMBC NMR spectra of the polar fraction of a CT tumour from a mouse infused for three hours with amido-15N-glutamine. Shows the regions of interest (R.O.I) of the signals from the indicated metabolites as the results of the 15N-incorporation from the amide group of glutamine during in vivo infusions. c, top panel: quantification of the [amido-15N]glutamine-derived enrichment of different metabolites, including nucleosides, in tumours from mice infused with amido-15N-glutamine (n = 3 mice per group); LC-MS; and bottom panel: cells isolated from the tumours and incubated with 2 mM amido-[15N]glutamine for 48 h (n = 3 independent experiments); LC-MS. Data are presented as mean ± S.D. Statistical analysis was performed using a two-tailed Student’s t-test. Complete list of exact P values is provided as a source data file. d, 1H-15N 2D-HMBC NMR spectra of the serum of mice infused with amido-[15N]glutamine, demonstrating the presence of labelled amino acids. e, Representative region of the 1H-15N 2D-HMBC NMR spectra of CT MYC liver tumours from mice treated with the vehicle or 25 mg/kg of the pan-amidotransferase inhibitor DON, infused with amido-[15N]glutamine. Note the DON-dependent suppression of the 15N incorporation.
Extended Data Fig. 6 The effect of co-inhibiting glutaminases and amidotransferases on metabolism and tumour cell proliferation.
a–c, The effect of DON (50 mg/kg, 4 h) on either CT or Gls1KO/shGls2 tumours from animals treated prior to [U-13C]glutamine bolus: . a, Total concentration of glutamine-derived amino acids (n = 3 mice per group); b, Total concentration of Krebs cycle intermediates (n = 3 mice per group); c, 2D 1H-13C-HSQC (heteronuclear single quantum coherence spectroscopy) NMR signals of glutamine and glutamate. d, Enrichment from either [U-13C]glutamine or [U-13C]glucose in HCCMYC-CT and HCCMYC-Gls1KO/shGls2 tumour cells treated with DON for 3 h (n = 3 independent experiments). GC-MS. e, Isotopologue distribution of the 13C incorporation into malate in the experiment shown in (d) (n = 3 independent experiments). Data are presented as mean ± S.D. Statistical analysis was performed using a two-tailed Student’s t-test. Complete list of exact P values is provided as a source data file. f, Combination of glutaminase inhibition and DON on cell proliferation of HCCMYC cells: HCCMYC-CT cells were treated with 1 μM CB-839 and/or 2 μM of DON. g, h, HCCMYC-Gls1KO/shGls2 (g) and HCCMYC-CT (h) cells treated with a combination of DON and CB-839 with or without the addition of the indicated amino acids. AAAP - a mix of alanine, aspartate, asparagine and proline. i, HepG2 cells treated with DON and/or CB-839 at the indicated concentrations. In (f–i) growth was monitored in an IncuCyte Live-Cell analysis system. In (f–i), representative curves from one of three independent experiments with 3 replicates are shown. Data are presented as mean ± S.D.
Extended Data Fig. 7 Effects of the simultaneous reduction of glycolysis and glutaminolysis on metabolism of tumours.
a, 13C-enrichment of glucose in the serum of mice bearing either CT and Hk2KO tumours administered [U-13C]glucose bolus (n = 6 mice per group). b, c, Total level of glucose 6P and fructose 6P (b) and Krebs cycle intermediates (c) in CT and Hk2KO tumours (n = 6 mice per group). GC-MS. d-h, Total metabolite levels in CT and Gls1KO/Hk2KO tumours (CT n = 8 mice; Gls1KO/Hk2KO n = 9 mice); LC-MS: d, additional Krebs cycle intermediates to those shown in Fig. 5f; e, glutamine; f, glycolytic intermediates; g, NEAAs; h, pentose phosphate pathway intermediates. i, j, Western blot of glutaminase and hexokinase isoform expression in CT and Gls1KO/Hk2KO tumours. β-actin was used as a loading control: i, Demonstration of the deletion of Gls1 and Hk2 (normal livers n = 2 mice, CT tumours n = 5 mice, Gls1KO/Hk2KO tumours n = 5 mice); j, Protein levels of other glutaminase and hexokinase isoforms in CT and Gls1KO/Hk2KO tumours (normal livers n = 2 mice, CT tumours n = 6 mice, Gls1KO/Hk2KO tumours n = 6 mice). Data are presented as mean ± S.D. Statistical analysis was performed using a two-tailed Student’s t-test. Complete list of exact P values is provided as a source data file.
Extended Data Fig. 8 Inhibition of lipid metabolism in MYC liver tumours.
a, 13C-enrichment of fatty acids (total fraction) from the indicated tissues of mice infused with [U-13C]glucose demonstrating a total blockade in fatty acid biosynthesis in FasnKO tumours (normal livers n = 4 mice, CT tumours n = 5 mice, FasnKO tumours n = 5 mice). A representative chromatogram of the fatty acid composition of a FasnKO tumour is shown. For unsaturated fatty acids, a residual enrichment that remained in the authentic standards after substraction of the natural abundance of 13C, was also substracted from the samples. b, Total fatty acid content in CT (n = 11 mice) and FasnKO (n = 10 mice) tumours. (c) Fatty acid ratios in CT (n = 11 mice) and FasnKO (n = 10 mice) tumours. d, Left panel, a heat map demonstrating the expression of genes involved in lipid transport in MYC-driven liver tumours compared to normal livers (gene microarray, Log2-transformed; n=4 mice per group). Right panel, absolute values are shown to identify those genes with higher total expression level, and to discriminate the genes with low levels of expression, which could be insufficient to produce relevant protein levels. Based on the expression of the relevant control genes (examples are shown in the lower panel) we considered 6 as a baseline level, while higher than 8 as a high level of expression. e, Total fatty acid content in CT (n = 6 mice) and FasnKO (n = 11 mice) tumours kept on the Low-Fat diet. f, The ratios of different fatty acids in CT (n = 6 mice) and FasnKO (n = 11 mice) tumours kept on the low-fat diet. Data are presented as mean ± S.D. Statistical analysis was performed using a two-tailed Student’s t-test. Complete list of exact P values is provided as a source data file. g, Light microscopy images of living cells isolated from CT and FasnKO tumours, after being maintained in the indicated media conditions with modulation of the lipid availability for 72 h. Representative images from three independent experiments are shown.
Extended Data Fig. 9 Inhibition of serine and glycine metabolism in MYC liver tumours.
a, Representative 15N-HMBC 2D NMR signals of the indicated metabolites in mouse livers and tumours after an [amino-15N]glutamine bolus (NMR spectra acquired: n = 4 mice per group). b, 15N-enrichment of glutamine (m+1) in the serum of mice shown in Fig. 7g and Extended Data Fig. 9a,d,e (CT/control diet n = 5 mice, CT/-SG diet n = 5 mice, Psat1KO/control diet n = 7, Psat1KO/-SG diet n = 7). GC-MS. Glutamine enrichment is estimated from quantification of its spontaneous product pyroglutamate. c, Psat1fl/fl cells were transduced with either MSCV-CreER or Empty vector, and both lines were treated with 4OH-Tamoxifen to induce Cre activity. The resulting Psat1WT and Psat1KO cells were cultured in DMEM with dialysed FCS and indicated metabolites. Ser: 0.5 mM serine; Gly: 0.5 mM glycine; 0.5 mM formate. Representative curves from one of three independent experiments with three replicates are shown. Data represent mean ± S.D. d, Total concentration of metabolites in livers and tumours of mice from the experiment shown in Fig. 7f,g and Extended data Fig. 9a,b,e (normal livers/control diet n = 4 mice, normal livers/-SG diet n = 4 mice, CT tumours/control diet n = 5 mice, CT tumours/-SG diet n = 5 mice, Psat1KO tumours/control diet n = 7 mice, Psat1KO tumours/-SG diet n = 7 mice). LC-MS. e, Total concentration and 15N-enrichment from an [amino-15N]glutamine bolus in serine and glycine from the serums of the indicated mice from the experiment shown in (a) (CT/control diet n = 5 mice, CT/-SG diet n = 5 mice, Psat1KO/control diet n =7 mice, Psat1KO/-SG diet n = 7). In (b and e) data are presented as mean ± S.D. Statistical analysis was performed using a two-tailed Student’s t-test. Complete list of exact P values is provided as a source data file. In (d) data are presented as mean ± S.D. Statistical analysis was performed using one-way ANOVA followed by Tukey's post hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001, with respect to CT tumours on control diet; #, P < 0.05; ##, P < 0.01; ###, P < 0.001, with respect to CT tumours on –SG diet.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed western blots
Source Data Fig. 1
Statistical source data
Source Data Fig. 2
Unprocessed western blots
Source Data Fig. 2
Statistical source data
Source Data Fig. 3
Statistical source data
Source Data Fig. 4
Statistical source data
Source Data Fig. 5
Unprocessed western blots
Source Data Fig. 5
Statistical source data
Source Data Fig. 6
Unprocessed western blots
Source Data Fig. 6
Statistical source data
Source Data Fig. 7
Unprocessed western blots
Source Data Fig. 7
Statistical source data
Source Data Extended Data Fig. 1
Statistical source data
Source Data Extended Data Fig. 3
Unprocessed western blots
Source Data Extended Data Fig. 3
Statistical source data
Source Data Extended Data Fig. 4
Statistical source data
Source Data Extended Data Fig. 5
Statistical source data
Source Data Extended Data Fig. 6
Statistical source data
Source Data Extended Data Fig. 7
Unprocessed western blots
Source Data Extended Data Fig. 7
Statistical source data
Source Data Extended Data Fig. 8
Statistical source data
Source Data Extended Data Fig. 9
Statistical source data
Rights and permissions
About this article
Cite this article
Méndez-Lucas, A., Lin, W., Driscoll, P.C. et al. Identifying strategies to target the metabolic flexibility of tumours. Nat Metab 2, 335–350 (2020). https://doi.org/10.1038/s42255-020-0195-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0195-8
This article is cited by
-
AURKA emerges as a vulnerable target for KEAP1-deficient non-small cell lung cancer by activation of asparagine synthesis
Cell Death & Disease (2024)
-
Filament formation drives catalysis by glutaminase enzymes important in cancer progression
Nature Communications (2024)
-
Aspirin reprogrammes colorectal cancer cell metabolism and sensitises to glutaminase inhibition
Cancer & Metabolism (2023)
-
Metabolic pathway analysis using stable isotopes in patients with cancer
Nature Reviews Cancer (2023)
-
Phenotypic profiling of solute carriers characterizes serine transport in cancer
Nature Metabolism (2023)