Abstract
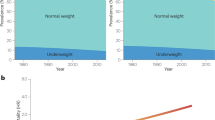
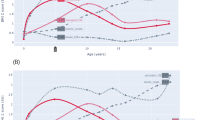
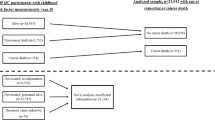
Childhood obesity is one of the most serious global public-health challenges of the twenty-first century. Over the past four decades, the number of children and adolescents with obesity has risen more than tenfold. Worldwide, an increasing number of youth are facing greater exposure to obesity throughout their lives, and this increase will contribute to the early development of type 2 diabetes, fatty liver and cardiovascular complications. Herein, we provide a brief overview of trends in the global shifts in, and environmental and genetic determinants of, childhood obesity. We then discuss recent progress in the elucidation of the central role of insulin resistance, the key element linking obesity and cardiovascular-risk-factor clustering, and the potential mechanisms through which ectopic lipid accumulation leads to insulin resistance and its associated cardiometabolic complications in obese adolescents. In the absence of effective prevention and intervention programs, childhood obesity will have severe public-health consequences for decades to come.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Consideration of the Evidence on Childhood Obesity for the Commission on Ending Childhood Obesity: Report of the Ad Hoc Working Group on Science and Evidence for Ending Childhood Obesity, Geneva, Switzerland (World Health Organization, 2016).
Lobstein, T., Baur, L. & Uauy, R. Obesity in children and young people: a crisis in public health. Obes. Rev. 5 (Suppl. 1), 4–104 (2004).
Prevalence of obesity. World Obesity Federation https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity (2015).
Wang, Y. & Lobstein, T. Worldwide trends in childhood overweight and obesity. Int. J. Pediatr. Obes. 1, 11–25 (2006).
Ogden, C. L., Carroll, M. D., Kit, B. K. & Flegal, K. M. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 311, 806–814 (2014).
Koplan, J. P. & Dietz, W. H. Caloric imbalance and public health policy. JAMA 282, 1579–1581 (1999).
Styne, D. M. et al. Pediatric obesity—assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 102, 709–757 (2017).
Freedman, D. S. & Sherry, B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 124 (Suppl. 1), S23–S34 (2009).
Freedman, D. S. et al. Classification of body fatness by body mass index-for-age categories among children. Arch. Pediatr. Adolesc. Med. 163, 805–811 (2009).
Cole, T. J. & Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 7, 284–294 (2012).
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390, 2627–2642 (2017).
National Center for Health Statistics. National Health Examination Surveys II (ages 6–11) and III (ages 12–17), and National Health and Nutrition Examination Surveys I, II and III, and 1999–2006 Centers for Disease Control and Prevention https://www.cdc.gov/nchs/nhanes/index.htm (2020).
Yanovski, J. A. Trends in underweight and obesity—scale of the problem. Nat. Rev. Endocrinol. 14, 5–6 (2018).
Fryar. C. D., Carroll, M. D. & Ogden, C. L. Prevalence of Overweight, Obesity, and Severe Obesity Among Children and Adolescents Aged 2–19 Years: United States, 1963–1965 Through 2015–2016 (National Center for Health Statistics, 2018); https://www.cdc.gov/nchs/data/hestat/obesity_child_15_16/obesity_child_15_16.pdf
Skinner, A. C. & Skelton, J. A. Prevalence and trends in obesity and severe obesity among children in the United States, 1999-2012. JAMA Pediatr. 168, 561–566 (2014).
Skinner, A. C., Perrin, E. M., Moss, L. A. & Skelton, J. A. Cardiometabolic risks and severity of obesity in children and young adults. N. Engl. J. Med. 373, 1307–1317 (2015).
Skinner, A. C., Ravanbakht, S. N., Skelton, J. A., Perrin, E. M. & Armstrong, S. C. Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics 141, e20173459 (2018).
Grossman, D. C. et al. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA 317, 2417–2426 (2017).
Jeffery, R. W. & Utter, J. The changing environment and population obesity in the United States. Obes. Res. 11 (Suppl.), 12S–22S (2003).
Ebbeling, C. B., Pawlak, D. B. & Ludwig, D. S. Childhood obesity: public-health crisis, common sense cure. Lancet 360, 473–482 (2002).
Campbell, K. J. et al. Associations between the home food environment and obesity-promoting eating behaviors in adolescence. Obesity (Silver Spring) 15, 719–730 (2007).
Gluckman, P., Nishtar, S. & Armstrong, T. Ending childhood obesity: a multidimensional challenge. Lancet 385, 1048–1050 (2015).
Jastreboff, A. M. et al. Altered brain response to drinking glucose and fructose in obese adolescents. Diabetes 65, 1929–1939 (2016).
Jastreboff, A. M. et al. Leptin is associated with exaggerated brain reward and emotion responses to food images in adolescent obesity. Diabetes Care 37, 3061–3068 (2014).
Brook, C. G., Huntley, R. M. & Slack, J. Influence of heredity and environment in determination of skinfold thickness in children. Br. Med. J. 2, 719–721 (1975).
Stunkard, A. J., Foch, T. T. & Hrubec, Z. A twin study of human obesity. JAMA 256, 51–54 (1986).
Stunkard, A. J., Harris, J. R., Pedersen, N. L. & McClearn, G. E. The body-mass index of twins who have been reared apart. N. Engl. J. Med. 322, 1483–1487 (1990).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387, 903–908 (1997).
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Farooqi, I. S. et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Invest. 106, 271–279 (2000).
Farooqi, I. S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348, 1085–1095 (2003).
Miraglia Del Giudice, E. et al. Low frequency of melanocortin-4 receptor (MC4R) mutations in a Mediterranean population with early-onset obesity. Int. J. Obes. Relat. Metab. Disord. 26, 647–651 (2002).
Santoro, N. et al. Prevalence of pathogenetic MC4R mutations in Italian children with early onset obesity, tall stature and familial history of obesity. BMC Med. Genet. 10, 25 (2009).
Clément, K. et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 24, 551–555 (2018).
Collet, T. H. et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol. Metab. 6, 1321–1329 (2017).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894 (2007).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206 (2015).
Shungin, D. et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 518, 187–196 (2015).
Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28, 166–174 (2019).
Felix, J. F. et al. Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum. Mol. Genet. 25, 389–403 (2016).
Bradfield, J. P. et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat. Genet. 44, 526–531 (2012).
Zhao, J. et al. The role of obesity-associated loci identified in genome-wide association studies in the determination of pediatric BMI. Obesity (Silver Spring) 17, 2254–2257 (2009).
Zhao, J. et al. Role of BMI-associated loci identified in GWAS meta-analyses in the context of common childhood obesity in European Americans. Obesity (Silver Spring) 19, 2436–2439 (2011).
Bradfield, J. P. et al. A trans-ancestral meta-analysis of genome-wide association studies reveals loci associated with childhood obesity. Hum. Mol. Genet. 28, 3327–3338 (2019).
Khera, A. V. et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell 177, 587–596.e9 (2019).
Turcot, V. et al. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat. Genet. 50, 26–41 (2018).
Dina, C. et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 39, 724–726 (2007).
Lauria, F. et al. Prospective analysis of the association of a common variant of FTO (rs9939609) with adiposity in children: results of the IDEFICS study. PLoS One 7, e48876 (2012).
Wardle, J. et al. Obesity associated genetic variation in FTO is associated with diminished satiety. J. Clin. Endocrinol. Metab. 93, 3640–3643 (2008).
den Hoed, M., Westerterp-Plantenga, M. S., Bouwman, F. G., Mariman, E. C. & Westerterp, K. R. Postprandial responses in hunger and satiety are associated with the rs9939609 single nucleotide polymorphism in FTO. Am. J. Clin. Nutr. 90, 1426–1432 (2009).
McTaggart, J. S. et al. FTO is expressed in neurones throughout the brain and its expression is unaltered by fasting. PLoS One 6, e27968 (2011).
Olszewski, P. K. et al. Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC Neurosci. 10, 129 (2009).
Fredriksson, R. et al. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology 149, 2062–2071 (2008).
Cowley, M. A. et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661 (2003).
Cecil, J. E., Tavendale, R., Watt, P., Hetherington, M. M. & Palmer, C. N. An obesity-associated FTO gene variant and increased energy intake in children. N. Engl. J. Med. 359, 2558–2566 (2008).
Qi, Q. et al. Fried food consumption, genetic risk, and body mass index: gene-diet interaction analysis in three US cohort studies. BMJ 348, g1610 (2014).
Karra, E. et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J. Clin. Invest. 123, 3539–3551 (2013).
Ranzenhofer, L. M. et al. The FTO gene and measured food intake in 5- to 10-year-old children without obesity. Obesity (Silver Spring) 27, 1023–1029 (2019).
Claussnitzer, M. et al. FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 373, 895–907 (2015).
Melhorn, S. J. et al. FTO genotype impacts food intake and corticolimbic activation. Am. J. Clin. Nutr. 107, 145–154 (2018).
Angulo, M. A., Butler, M. G. & Cataletto, M. E. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J. Endocrinol. Invest. 38, 1249–1263 (2015).
Burnett, L. C. et al. Deficiency in prohormone convertase PC1 impairs prohormone processing in Prader-Willi syndrome. J. Clin. Invest. 127, 293–305 (2017).
Jackson, R. S. et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat. Genet. 16, 303–306 (1997).
Paisey, R.B. et al. in GeneReviews (eds Adam, M. P. et al.) (University of Washington, 1993).
Han, J. C. et al. Comprehensive endocrine-metabolic evaluation of patients with Alström syndrome compared with BMI-matched controls. J. Clin. Endocrinol. Metab. 103, 2707–2719 (2018).
Forsythe, E., Kenny, J., Bacchelli, C. & Beales, P. L. Managing Bardet-Biedl Syndrome: now and in the future. Front Pediatr. 6, 23 (2018).
Sherafat-Kazemzadeh, R. et al. Hyperphagia among patients with Bardet-Biedl syndrome. Pediatr. Obes. 8, e64–e67 (2013).
Feuillan, P. P. et al. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. J. Clin. Endocrinol. Metab. 96, E528–E535 (2011).
Daniels, S. R. et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation 111, 1999–2012 (2005).
Morrison, J. A., Barton, B. A., Biro, F. M., Daniels, S. R. & Sprecher, D. L. Overweight, fat patterning, and cardiovascular disease risk factors in black and white boys. J. Pediatr. 135, 451–457 (1999).
Morrison, J. A., Sprecher, D. L., Barton, B. A., Waclawiw, M. A. & Daniels, S. R. Overweight, fat patterning, and cardiovascular disease risk factors in black and white girls: The National Heart, Lung, and Blood Institute Growth and Health Study. J. Pediatr. 135, 458–464 (1999).
Pinhas-Hamiel, O. et al. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J. Pediatr. 128, 608–615 (1996).
Weiss, R. et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 350, 2362–2374 (2004).
Berenson, G. S. et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults: the Bogalusa Heart Study. N. Engl. J. Med. 338, 1650–1656 (1998).
Freedman, D. S. et al. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics 115, 22–27 (2005).
Juonala, M. et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 365, 1876–1885 (2011).
Ludwig, D. S. Childhood obesity: the shape of things to come. N. Engl. J. Med. 357, 2325–2327 (2007).
Wajchenberg, B. L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 21, 697–738 (2000).
Frayn, K. N. Adipose tissue as a buffer for daily lipid flux. Diabetologia 45, 1201–1210 (2002).
Smith, S. R. et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 50, 425–435 (2001).
Taksali, S. E. et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes 57, 367–371 (2008).
Shulman, G. I. Cellular mechanisms of insulin resistance. J. Clin. Invest. 106, 171–176 (2000).
Shulman, G. I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 371, 1131–1141 (2014).
Gray, S. L. & Vidal-Puig, A. J. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr. Rev. 65, S7–S12 (2007).
Virtue, S. & Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome: an allostatic perspective. Biochim. Biophys. Acta 1801, 338–349 (2010).
Kursawe, R. et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes 59, 2288–2296 (2010).
Kursawe, R. et al. Decreased transcription of ChREBP-α/β isoforms in abdominal subcutaneous adipose tissue of obese adolescents with prediabetes or early type 2 diabetes: associations with insulin resistance and hyperglycemia. Diabetes 62, 837–844 (2013).
Gillum, M. P. et al. SirT1 regulates adipose tissue inflammation. Diabetes 60, 3235–3245 (2011).
Nouws, J. et al. Altered in vivo lipid fluxes and cell dynamics in subcutaneous adipose tissues are associated with the unfavourable pattern of fat distribution in obese adolescent girls. Diabetes 68, 1168–1177 (2019).
Petersen, M. C. & Shulman, G. I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98, 2133–2223 (2018).
Reaven, G. Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease. Circulation 106, 286–288 (2002).
Roden, M. et al. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Invest. 97, 2859–2865 (1996).
Weiss, R. et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 362, 951–957 (2003).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).
Nagarajan, A. et al. MARCH1 regulates insulin sensitivity by controlling cell surface insulin receptor levels. Nat. Commun. 7, 12639 (2016).
Cali, A. M. et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology 49, 1896–1903 (2009).
Hershkop, K. et al. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J. Clin. Endocrinol. Metab. 101, 2423–2431 (2016).
Weiss, R. et al. Degree of obesity and glucose allostasis are major effectors of glucose tolerance dynamics in obese youth. Diabetes Care 30, 1845–1850 (2007).
Giannini, C. et al. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes 61, 606–614 (2012).
Cali, A. M. et al. Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents: a perfect proatherogenic state. Diabetes Care 30, 3093–3098 (2007).
Caprio, S., Perry, R. & Kursawe, R. Adolescent obesity and insulin resistance: roles of ectopic fat accumulation and adipose inflammation. Gastroenterology 152, 1638–1646 (2017).
Winer, J. C. et al. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J. Clin. Endocrinol. Metab. 91, 4415–4423 (2006).
Brady, T. M. The role of obesity in the development of left ventricular hypertrophy among children and adolescents. Curr. Hypertens. Rep. 18, 3 (2016).
Reinehr, T., Kiess, W., de Sousa, G., Stoffel-Wagner, B. & Wunsch, R. Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism 55, 113–118 (2006).
Yajnik, C. S. et al. Higher glucose, insulin and insulin resistance (HOMA-IR) in childhood predict adverse cardiovascular risk in early adulthood: the Pune Children’s Study. Diabetologia 58, 1626–1636 (2015).
Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 346, 1221–1231 (2002).
Schwimmer, J. B. et al. Prevalence of fatty liver in children and adolescents. Pediatrics 118, 1388–1393 (2006).
Tricò, D. et al. Metabolic features of non-alcoholic fatty liver (NAFL) in obese adolescents: findings from a multi-ethnic cohort. Hepatology 68, 1376–1390 (2018).
Feldstein, A. E. et al. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut 58, 1538–1544 (2009).
D’Adamo, E. et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 33, 1817–1822 (2010).
Newton, K. P. et al. Prevalence of prediabetes and type 2 diabetes in children with non-alcoholic fatty liver disease. JAMA Pediatr. 170, e161971 (2016).
Schwimmer, J. B., Pardee, P. E., Lavine, J. E., Blumkin, A. K. & Cook, S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation 118, 277–283 (2008).
Wagenknecht, L. E. et al. Correlates and heritability of nonalcoholic fatty liver disease in a minority cohort. Obesity (Silver Spring) 17, 1240–1246 (2009).
Schwimmer, J. B. et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology 136, 1585–1592 (2009).
Loomba, R. et al. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology 149, 1784–1793 (2015).
Romeo, S. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465 (2008).
Mitsche, M. A., Hobbs, H. H. & Cohen, J. C. Patatin-like phospholipase domain–containing protein 3 promotes transfer of essential fatty acids from triglycerides to phospholipids in hepatic lipid droplets. J. Biol. Chem. 293, 6958–6968 (2018).
Tian, C., Stokowski, R. P., Kershenobich, D., Ballinger, D. G. & Hinds, D. A. Variant in PNPLA3 is associated with alcoholic liver disease. Nat. Genet. 42, 21–23 (2010).
Núñez-Torres, R. et al. The PNPLA3 genetic variant rs738409 influences the progression to cirrhosis in HIV/hepatitis C virus coinfected patients. PLoS One 11, e0168265 (2016).
Santoro, N. et al. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology 55, 781–789 (2012).
Goffredo, M. et al. Role of TM6SF2 rs58542926 in the pathogenesis of nonalcoholic pediatric fatty liver disease: a multiethnic study. Hepatology 63, 117–125 (2016).
Santoro, N. et al. Hepatic de novo lipogenesis in obese youth is modulated by a common variant in the GCKR gene. J. Clin. Endocrinol. Metab. 100, E1125–E1132 (2015).
Stender, S. et al. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat. Genet. 49, 842–847 (2017).
Freedman, D. S., Mei, Z., Srinivasan, S. R., Berenson, G. S. & Dietz, W. H. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J. Pediatr. 150, 12–17.e2 (2007).
Zabarsky, G. et al. Impact of severe obesity on cardiovascular risk factors in youth. J. Pediatr. 192, 105–114 (2018).
Al-Khudairy, L. et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst. Rev. 6, CD012691 (2017).
Rajjo, T. et al. The association of weight loss and cardiometabolic outcomes in obese children: systematic review and meta-regression. J. Clin. Endocrinol. Metab. 102, 758–762 (2017).
Fonvig, C. E. et al. Multidisciplinary care of obese children and adolescents for one year reduces ectopic fat content in liver and skeletal muscle. BMC Pediatr. 15, 196 (2015).
Kloppenborg, J. T. et al. The effect of impaired glucose metabolism on weight loss in multidisciplinary childhood obesity treatment. Pediatr. Diabetes 19, 366–374 (2018).
Farpour-Lambert, N. J. et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J. Am. Coll. Cardiol. 54, 2396–2406 (2009).
Reinehr, T. et al. Which amount of BMI-SDS reduction is necessary to improve cardiovascular risk factors in overweight children? J. Clin. Endocrinol. Metab. 101, 3171–3179 (2016).
Reinehr, T., Kleber, M. & Toschke, A. M. Lifestyle intervention in obese children is associated with a decrease of the metabolic syndrome prevalence. Atherosclerosis 207, 174–180 (2009).
Savoye, M. et al. Long-term results of an obesity program in an ethnically diverse pediatric population. Pediatrics 127, 402–410 (2011).
Adam, T. C. et al. Insulin sensitivity as an independent predictor of fat mass gain in Hispanic adolescents. Diabetes Care 32, 2114–2115 (2009).
Weiss, R. et al. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care 28, 902–909 (2005).
van der Baan-Slootweg, O. et al. Inpatient treatment of children and adolescents with severe obesity in the Netherlands: a randomized clinical trial. JAMA Pediatr. 168, 807–814 (2014).
Zeller, M. et al. Predictors of attrition from a pediatric weight management program. J. Pediatr. 144, 466–470 (2004).
Hampl, S., Paves, H., Laubscher, K. & Eneli, I. Patient engagement and attrition in pediatric obesity clinics and programs: results and recommendations. Pediatrics 128 (Suppl. 2), S59–S64 (2011).
Guerciolini, R. Mode of action of orlistat. Int. J. Obes. Relat. Metab. Disord. 21 (Suppl. 3), S12–S23 (1997).
Chanoine, J. P., Hampl, S., Jensen, C., Boldrin, M. & Hauptman, J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA 293, 2873–2883 (2005).
Klein, D. J. et al. Liraglutide’s safety, tolerability, pharmacokinetics, and pharmacodynamics in pediatric type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Technol. Ther. 16, 679–687 (2014).
Mastrandrea, L. D. et al. Liraglutide effects in a paediatric (7-11 y) population with obesity: a randomized, double-blind, placebo-controlled, short-term trial to assess safety, tolerability, pharmacokinetics, and pharmacodynamics. Pediatr. Obes. 14, e12495 (2019).
Secher, A. et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest. 124, 4473–4488 (2014).
Kühnen, P. et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 375, 240–246 (2016).
Cali, A. M. et al. Rosiglitazone improves glucose metabolism in obese adolescents with impaired glucose tolerance: a pilot study. Obesity (Silver Spring) 19, 94–99 (2011).
Pratt, J. S. A. et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg. Obes. Relat. Dis. 14, 882–890 (2018).
Inge, T. H. et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N. Engl. J. Med. 374, 113–123 (2016).
Inge, T. H. et al. Five-year outcomes of gastric bypass in adolescents as compared with adults. N. Engl. J. Med. 380, 2136–2145 (2019).
Olbers, T. et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. 5, 174–183 (2017).
Dewberry, L. C. et al. Change in gastrointestinal symptoms over the first 5 years after bariatric surgery in a multicenter cohort of adolescents. J. Pediatr. Surg. 54, 1220–1225 (2019).
Zeller, M. H. et al. Severe obesity and comorbid condition impact on the weight-related quality of life of the adolescent patient. J. Pediatr. 166, 651–659.e4 (2015).
Ells, L.J. et al. Surgery for the treatment of obesity in children and adolescents. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD011740 (2015).
Yu, E. W. et al. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J. Clin. Endocrinol. Metab. 100, 1452–1459 (2015).
Lu, C. W. et al. Fracture risk after bariatric surgery: a 12-year nationwide cohort study. Medicine (Baltimore) 94, e2087 (2015).
Denzer, C., Reithofer, E., Wabitsch, M. & Widhalm, K. The outcome of childhood obesity management depends highly upon patient compliance. Eur. J. Pediatr. 163, 99–104 (2004).
Rankin, J. et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc. Health Med. Ther. 7, 125–146 (2016).
Beamish, A. J. & Reinehr, T. Should bariatric surgery be performed in adolescents? Eur. J. Endocrinol. 176, D1–D15 (2017).
Scheimann, A. O., Butler, M. G., Gourash, L., Cuffari, C. & Klish, W. Critical analysis of bariatric procedures in Prader-Willi syndrome. J. Pediatr. Gastroenterol. Nutr. 46, 80–83 (2008).
Bretault, M. et al. Clinical review: bariatric surgery following treatment for craniopharyngioma: a systematic review and individual-level data meta-analysis. J. Clin. Endocrinol. Metab. 98, 2239–2246 (2013).
Rodgers, A., Woodward, A., Swinburn, B. & Dietz, W. H. Prevalence trends tell us what did not precipitate the US obesity epidemic. Lancet Public Health 3, e162–e163 (2018).
Young, L. R. & Nestle, M. Expanding portion sizes in the US marketplace: implications for nutrition counseling. J. Am. Diet. Assoc. 103, 231–234 (2003).
Bray, G. A., Nielsen, S. J. & Popkin, B. M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 79, 537–543 (2004).
Population-based Approaches to Childhood Obesity Prevention (World Health Organization, 2012).
Report of the Commission on Ending Childhood Obesity (World Health Organization, 2016); https://www.who.int/end-childhood-obesity/publications/echo-report/en/.
Institute of Medicine. Early Childhood Obesity Prevention Policies (National Academy of Sciences, 2011); http://www.nationalacademies.org/hmd/Reports/2011/Early-Childhood-Obesity-Prevention-Policies.aspx
Roberto, C. A. et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet 385, 2400–2409 (2015).
Swinburn, B. A. et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 378, 804–814 (2011).
Stuckler, D. & Nestle, M. Big food, food systems, and global health. PLoS Med. 9, e1001242 (2012).
Ward, Z. J. et al. Simulation of growth trajectories of childhood obesity into adulthood. N. Engl. J. Med. 377, 2145–2153 (2017).
Santoro, N. & Caprio, S. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis in obese adolescents: a looming marker of cardiac dysfunction. Hepatology 59, 372–374 (2014).
Acknowledgements
The authors are grateful to B. Pierpont and M. Savoye for their support and dedication to this work. S.C. is supported by US National Institutes of Health (NIH) grants R01-DK111038 and R01-HD028016. N.S. is supported by NIH grant R01-DK114504. The work of S.C. and N.S. at Yale is also made possible by NIH grant P30DK045735. This publication was also made possible by CTSA grant UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), a component of the NIH. The contents herein are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Author information
Authors and Affiliations
Contributions
S.C. drafted the following: Abstract, introduction, ‘Epidemiology’, ‘Environmental determinants’, ‘The effects of the FTO genotype on food intake in children’, ‘Future outlook’, ‘Ectopic fat storage in obese youth’ and ‘Halting the epidemic of childhood obesity’. R.W. drafted the following: ‘Bariatric surgery in paediatric obesity’ and ‘Obesity dynamics and CVRF stability in obese adolescents’. N.S. drafted the following: ‘Genetics of childhood obesity’, ‘Syndromic forms of obesity’, ‘Non-alcoholic fatty liver disease in obese youth’ and ‘Pharmacological approaches’. All the authors have read and edited the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Elena Bellafante.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Caprio, S., Santoro, N. & Weiss, R. Childhood obesity and the associated rise in cardiometabolic complications. Nat Metab 2, 223–232 (2020). https://doi.org/10.1038/s42255-020-0183-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0183-z
This article is cited by
-
Mental health in children and adolescents with overweight or obesity
BMC Public Health (2023)
-
Serum endocan as a predictive biomarker of cardiovascular risk in obese pediatric patients
Italian Journal of Pediatrics (2023)
-
CETP and APOA2 polymorphisms are associated with weight loss and healthy eating behavior changes in response to digital lifestyle modifications
Scientific Reports (2023)
-
Pediatric dyslipidemia is associated with increased urinary ACE activity, blood pressure values, and carotidal-femoral pulse wave velocity
Hypertension Research (2023)
-
Epidemiology, mortality, and health service use of local-level multimorbidity patterns in South Spain
Nature Communications (2023)