Abstract
Critical to the bacterial stringent response is the rapid relocation of resources from proliferation toward stress survival through the respective accumulation and degradation of (p)ppGpp by RelA and SpoT homologues. While mammalian genomes encode MESH1, a homologue of the bacterial (p)ppGpp hydrolase SpoT, neither (p)ppGpp nor its synthetase has been identified in mammalian cells. Here, we show that human MESH1 is an efficient cytosolic NADPH phosphatase that facilitates ferroptosis. Visualization of the MESH1–NADPH crystal structure revealed a bona fide affinity for the NADPH substrate. Ferroptosis-inducing erastin or cystine deprivation elevates MESH1, whose overexpression depletes NADPH and sensitizes cells to ferroptosis, whereas MESH1 depletion promotes ferroptosis survival by sustaining the levels of NADPH and GSH and by reducing lipid peroxidation. The ferroptotic protection by MESH1 depletion is ablated by suppression of the cytosolic NAD(H) kinase, NADK, but not its mitochondrial counterpart NADK2. Collectively, these data shed light on the importance of cytosolic NADPH levels and their regulation under ferroptosis-inducing conditions in mammalian cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. The coordinates of the hMesh1 D66K–NADPH complex have been deposited in the Protein Data Bank under accession code 5VXA. The RNA-seq data have been deposited into NCBI GEO with accession number GSE114282. Source data for Figs. 1, 3 and 4 are presented with the paper.
References
Srivatsan, A. & Wang, J. D. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr. Opin. Microbiol. 11, 100–105 (2008).
Potrykus, K. & Cashel, M. (p)ppGpp: still magical? Annu. Rev. Microbiol. 62, 35–51 (2008).
Kriel, A. et al. Direct regulation of GTP homeostasis by (p)ppGpp: a critical component of viability and stress resistance. Mol. Cell 48, 231–241 (2012).
Sun, D. et al. A metazoan ortholog of SpoT hydrolyzes ppGpp and functions in starvation responses. Nat. Struct. Mol. Biol. 17, 1188–1194 (2010).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Hogg, T., Mechold, U., Malke, H., Cashel, M. & Hilgenfeld, R. Conformational antagonism between opposing active sites in a bifunctional RelA/SpoT homolog modulates (p)ppGpp metabolism during the stringent response [corrected]. Cell 117, 57–68 (2004).
Baykov, A. A., Evtushenko, O. A. & Avaeva, S. M. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal. Biochem. 171, 266–270 (1988).
Petheo, G. L. & Demaurex, N. Voltage- and NADPH-dependence of electron currents generated by the phagocytic NADPH oxidase. Biochem. J. 388, 485–491 (2005).
Clayton, D. A. & Shadel, G. S. Isolation of mitochondria from cells and tissues. Cold Spring Harb. Protoc. 2014, pdb.top074542 (2014).
Navas, P., Minnifield, N., Sun, I. & Morre, D. J. NADP phosphatase as a marker in free-flow electrophoretic separations for cisternae of the Golgi apparatus midregion. Biochim. Biophys. Acta 881, 1–9 (1986).
Pollak, N., Dolle, C. & Ziegler, M. The power to reduce: pyridine nucleotides—small molecules with a multitude of functions. Biochem. J. 402, 205–218 (2007).
Estrella, M. A. et al. The metabolites NADP(+) and NADPH are the targets of the circadian protein Nocturnin (Curled). Nat. Commun. 10, 2367 (2019).
Ding, C.-K. C.et al. Mammalian stringent-like response mediated by the cytosolic NADPH phosphatase MESH1. Preprint at bioRxiv https://doi.org/10.1101/325266 (2018).
Yang, W. S. & Stockwell, B. R. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 26, 165–176 (2016).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Chen, P. H. et al. Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism. Cell Death Differ. 27, 1008–1022 (2020).
Yang, W. H., Ding, C. K. C., Sun, T., Hsu, D. S. & Chi, J. T. The Hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep. 28, 2501–2508.e2504 (2019).
Yang, W. H. et al. A TAZ-ANGPTL4-NOX2 axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancer. Mol. Cancer Res. 18, 79–90 (2020).
Shimada, K., Hayano, M., Pagano, N. C. & Stockwell, B. R. Cell-line selectivity improves the predictive power of pharmacogenomic analyses and helps identify NADPH as biomarker for ferroptosis sensitivity. Cell Chem. Biol. 23, 225–235 (2016).
Ohashi, K., Kawai, S. & Murata, K. Identification and characterization of a human mitochondrial NAD kinase. Nat. Commun. 3, 1248 (2012).
Pollak, N., Niere, M. & Ziegler, M. NAD kinase levels control the NADPH concentration in human cells. J. Biol. Chem. 282, 33562–33571 (2007).
Lewis, C. A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Tarangelo, A. et al. p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep. 22, 569–575 (2018).
Skouta, R. et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 136, 4551–4556 (2014).
Angeli, J. P. F. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Du, K. et al. Inhibiting xCT/SLC7A11 induces ferroptosis of myofibroblastic hepatic stellate cells and protects against liver fibrosis. Preprint at bioRxiv https://doi.org/10.1101/2019.12.23.886259 (2019).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59, 298–308 (2015).
Linkermann, A. et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl Acad. Sci. USA 111, 16836–16841 (2014).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Yang, X. et al. A public genome-scale lentiviral expression library of human ORFs. Nat. Methods 8, 659–661 (2011).
Tang, X. et al. Comprehensive profiling of amino acid response uncovers unique methionine-deprived response dependent on intact creatine biosynthesis. PLoS Genet. 11, e1005158 (2015).
Tang, X. et al. Cystine deprivation triggers programmed necrosis in VHL-deficient renal cell carcinomas. Cancer Res. 76, 1892–1903 (2016).
Tang, X. et al. Cystine addiction of triple-negative breast cancer associated with EMT augmented death signaling. Oncogene 36, 4379 (2017).
Tao, R. et al. Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat. Methods 14, 720–728 (2017).
Acknowledgements
This work was supported in part by DOD grants (grant nos. W81XWH-17-1-0143, W81XWH-15-1-0486 and W81XWH-19-1-0842 to J.-T.C.), the Emerson Collective (to J.-T.C.), NIH grants no. R01GM124062 and R21AI149205 (to J.-T.C. and P.Z.) and no. R01GM115355 (to P.Z.), the Duke Bridge Fund, the Duke Cancer Institute (DCI) Discovery fund and the DCI Pilot fund. X-ray diffraction data were collected at the Southeast Regional Collaborative Access Team (SER-CAT) 22-BM beamline at the Advanced Photon Source, Argonne National Laboratory, supported by the US Department of Energy, Office of Science and the Office of Basic Energy Sciences under Contract no. W-31-109-Eng-38. We appreciate the great suggestions and help from the X. Shen laboratory.
Author information
Authors and Affiliations
Contributions
The experimental strategy was conceived by J.-T.C. and P.Z. and further developed by C.-K.C.D. and J.R. X-ray crystallography was conducted by J.R. and analysed by J.R. and P.Z. Enzymatic assays were performed and analysed by J.R., J.Z. and P.Z. Mass spectrometry measurements were carried out by Z.G. Measurements of the enzymatic activity and NADP(H) concentration in the cell lysates were carried out by C.-K.C.D., J.W. and J.R. Cell culture and transcriptome profiling experiments were carried out by C.-K.C.D. and K.-Y.C. with assistance from J.W., T.S., P.-H.C., E.X., S.T., H.L. and J.A. C.-C.L. contributed to the NADPH sensor and phenotype experiments. Lipid peroxidation measurements were carried out by W.-H.Y. C.-K.C.D., J.R., J.-T.C. and P.Z. wrote the manuscript with input from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: George Caputa.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
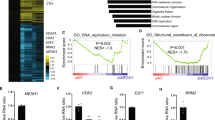
Extended Data Fig. 1 MESH1 contributes to the NADPH phosphatase activity in cells.
NADPH phosphatase activity of HEK-293T was estimated by incubating NADPH with cell lysates with different MESH1 status; phosphate release over time was measured by the malachite green assay and normalized by the total lysate protein (μmol/min/mg total lysate protein). a, Significantly decreased cellular NADPH phosphatase activity by silencing MESH1 in HEK-293T cells. b, Increased cellular NADPH phosphatase activity with overexpression of WT MESH1, but not with overexpression of enzymatically deficient MESH1 (MESH1-E65A) in HEK-293T cells. Black dots represent siNT or empty vector samples, blue dots represent siMESH1 samples, red dots represent MESH1-WT samples, and grey dots represent MESH1-E65A samples. Error bars represent s.d. (n=3 biologically independent samples per condition). Statistical analysis: one way ANOVA with Tukey’s HSD post-hoc, **P<0.01, N.S., not significant. Source data are provided in the Source Data File.
Extended Data Fig. 2 MESH1 asymmetric unit, omit map, and Zn2+ anomalous phasing.
a, Crystallographic asymmetric unit is shown with two protomers of hMESH1 in the ribbon diagram and NADPH molecules in the stick model. b, 2mFo-DFc omit maps for the ADP (left and middle) and nicotinamide riboside moieties (right) of the NADPH molecules contoured to 0.8σ in purple mesh. c, The active site of each protomer is shown along with density from Zn2+ anomalous scattering contoured to 6σ in black mesh.
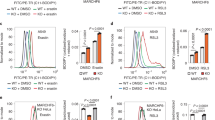
Extended Data Fig. 3 Up-regulation of MESH1 protein and its regulation of NADP(H) and cell survival during ferroptosis.
a, b, The levels of MESH1 protein were determined by Western blots of MESH1 and β-tubulin protein in RCC4 cells treated with (a) DMSO vs. erastin (3 µM; 12 h) or (b) 200 or 0 µM for 20 h. All the representative western blots were repeated for 3 biologically independent times with similar results. c, Overexpression of WT MESH1, but not the MESH1-E65A mutant, significantly reduced the intracellular NADP(H) content in RCC4 cells. d, Overexpression of MESH1-WT, but not MESH1-E65A, sensitized the cell death to erastin treatment in RCC4 cells. Black dots represent empty vector samples, red dots represent MESH1-WT samples, and grey dots represent MESH1-E65A samples. e, MESH1 silencing restored the intracellular NAPDH level decreased by erastin treatment. HEK-293T cells transfected with the NADPH fluorescent biosensor, iNAP1, and siNT or two independent siMESH1 RNAs were treated with 20 µM of erastin. The ratio of fluorescence excited at 420 nm and 485 nm indicated the cellular NADPH level. The fluorescence ratios of individual treatments were normalized to that of the control condition. (n=6 biologically independent samples). f, The viability of RCC4 cells transfected with siNT (black) or two independent siMESH1 (blue) and treated with indicated concentrations of erastin for one day. Each dot represents one sample; there are three biologically independent samples (n=3) in each group unless otherwise specified. Error bars indicate s.d. Statistical analysis: one-way ANOVA with Tukey’s HSD post-hoc test, *P<0.05, **P<0.01. Source data are provided in the Source Data File.
Extended Data Fig. 4 MESH1 depletion conferred resistance to ferroptosis in multiple assays.
a–c, Erastin-induced cell death is rescued by MESH1 silencing in RCC4 cells using multiple cell death and viability assays: a, Cytotox-Flour for measuring protease release in the media due to cell death; b, Crystal violet staining for attached viable cells; and c, CellTiter-Glo for intracellular ATP and viability. Error bars indicate s.d. (n=3 biologically independent samples per condition). Statistical analysis: one-way ANOVA with Tukey’s HSD post-hoc test, *P<0.05, **P<0.01. Source data are provided in the Source Data File.
Extended Data Fig. 5 MESH1 depletion increased resistance to ferroptosis in multiple cell lines.
a-h. Erastin-induced ferroptotic cell death of indicated cell lines was rescued by MESH1 silencing as accessed by CellTiter-Glo: a, HEK-293T cells exposed to erastin 2.5 μM for one day; b, lung adenocarcinoma cell line H1975 with erastin 10 μM for two days; c, breast cancer cell line MDA-MB-231 with erastin 2.5 μM for one day; d, prostate cancer cell line PC3 with erastin 10 μM for one day; e, fibrosarcoma cell line HT1080 with erastin 2.5 μM for one day; f, Ewing’s sarcoma cell line A673 with erastin 2.5 μM for one day; g, pancreatic carcinoma cell line PANC1 with erastin 2.5 μM for one day, and h, clear cell renal carcinoma cell line 786-O with erastin 5 μM for one day. Black dots represent siNT samples, blue dots represent siMESH1 samples. Error bars indicate s.d. (n=3 biologically independent samples per condition). Statistical analysis: one-way ANOVA with Tukey’s HSD post-hoc test, *P<0.05, **P<0.01. Source data are provided in the Source Data File.
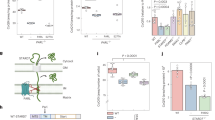
Extended Data Fig. 6 MESH1-depletion increased the ratio of reduced glutathione over oxidized glutathione.
a–d, The level of indicated metabolites in RCC4 transfected with non-targeting (NT) siRNA or MESH1-targeting siRNA were quantified by mass spectrometry and normalized by DNA content, a, cysteine; b, oxidized glutathione (GSSG); c, reduced glutathione (GSH) (effect size 1.746, p=0.0491); and d, normalized intensity ratio of GSH/GSSG. n=5 biologically independent samples per condition. Each box plot was defined by a lower line (at the 25th percentile), a central line (at the centre), and a higher line (at the 75th percentile). The whisker boundaries marked the minima and the maxima of the data. Statistical analysis: two-tail student’s t-test, *P<0.05. Source data are provided in the Source Data File.
Extended Data Fig. 7 MESH1 silenced cells were re-sensitized to erastin-induced cell death upon NADK silencing.
Percentage of cell viability of HEK-293T cells after treatment with erastin 10 μM for one day, showing simultaneously silencing MESH1 with NADK, but not NADK2, restored sensitivity to erastin-induced ferroptosis. Black dots represent siNT samples, blue dots represent siMESH1 samples. Error bars indicate s.d. (for siNT and siMESH1 n=6 biologically independent samples per condition; for other genetic conditions n=3 biologically independent samples). Statistical analysis: one-way ANOVA with Tukey’s HSD post-hoc test *P<0.05, **P<0.01. Source data are provided in the Source Data File.
Extended Data Fig. 8 Relative expression levels of genes involved in the NADPH and glutathione metabolisms.
Three plates of RCC4 cells were sequenced and the relative expression levels of the indicated genes were calculated by RPKM (Reads per kilo base per million mapped reads). Error bars indicate s.d. (n=3 biologically independent samples per condition).
Supplementary information
Supplementary Information
Supplementary Table 1
Supplementary Data 1
Chemical structure data.
Source data
Source Data Fig. 1
Unprocessed western blots and/or gels for Fig. 1g,h.
Source Data Fig. 1
Raw data and statistics for Fig. 1c,g.
Source Data Fig. 3
Unprocessed western blots and/or gels for Fig. 3c.
Source Data Fig. 3
Raw data and statistics for Fig. 3b,c,e–i.
Source Data Fig. 4
Unprocessed western blots and/or gels for Fig. 4a.
Source Data Fig. 4
Raw data and statistics for Fig. 4a–d.
Source Data Extended Data Fig. 1
Unprocessed western blots and/or gels for Fig. 1a,b.
Source Data Extended Data Fig. 1
Raw data and statistics for Extended Data Fig. 1a,b.
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels for Fig. 3a–d.
Source Data Extended Data Fig. 3
Raw data and statistics for Extended Data Fig. 3c–f.
Source Data Extended Data Fig. 4
Raw data and statistics for Extended Data Fig. 4a–c.
Source Data Extended Data Fig. 5
Raw data and statistics for Extended Data Fig. 5a–h.
Source Data Extended Data Fig. 6
Raw data and statistics for Extended Data Fig. 6a–d.
Source Data Extended Data Fig. 7
Raw data and statistics for Extended Data Fig. 7.
Rights and permissions
About this article
Cite this article
Ding, CK.C., Rose, J., Sun, T. et al. MESH1 is a cytosolic NADPH phosphatase that regulates ferroptosis. Nat Metab 2, 270–277 (2020). https://doi.org/10.1038/s42255-020-0181-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0181-1
This article is cited by
-
The cell biology of ferroptosis
Nature Reviews Molecular Cell Biology (2024)
-
Metabolism-regulated ferroptosis in cancer progression and therapy
Cell Death & Disease (2024)
-
Significance of the plastidial stringent response for plant growth on soil
Plant Growth Regulation (2024)
-
Combination of blockade of endothelin signalling and compensation of IGF1 expression protects the retina from degeneration
Cellular and Molecular Life Sciences (2024)
-
FSP1 oxidizes NADPH to suppress ferroptosis
Cell Research (2023)