Abstract
Decreased NAD+ levels have been shown to contribute to metabolic dysfunction during aging. NAD+ decline can be partially prevented by knockout of the enzyme CD38. However, it is not known how CD38 is regulated during aging, and how its ecto-enzymatic activity impacts NAD+ homeostasis. Here we show that an increase in CD38 in white adipose tissue (WAT) and the liver during aging is mediated by accumulation of CD38+ immune cells. Inflammation increases CD38 and decreases NAD+. In addition, senescent cells and their secreted signals promote accumulation of CD38+ cells in WAT, and ablation of senescent cells or their secretory phenotype decreases CD38, partially reversing NAD+ decline. Finally, blocking the ecto-enzymatic activity of CD38 can increase NAD+ through a nicotinamide mononucleotide (NMN)-dependent process. Our findings demonstrate that senescence-induced inflammation promotes accumulation of CD38 in immune cells that, through its ecto-enzymatic activity, decreases levels of NMN and NAD+.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
No custom codes or mathematical algorithms that are deemed central to the conclusions of our manuscript were used in our studies.
References
Hogan, K. A., Chini, C. C. S. & Chini, E. N. The multi-faceted ecto-enzyme CD38: roles in immunomodulation, cancer, aging and metabolic diseases. Front. Immunol. 10, https://doi.org/10.3389/fimmu.2019.01187 (2019).
Katsyuba, E., Romani, M., Hofer, D. & Auwerx, J. NAD+ homeostasis in health and disease. Nat. Metab. 2, 9–31 (2020).
Johnson, S. & Imai, S.-I. NAD+ biosynthesis, aging and disease. F1000Res 7, 132 (2018).
McReynolds, M. R., Chellappa, K. & Baur, J. A. Age-related NAD+ decline. Exp. Gerontol. 134, 110888 (2020).
Camacho-Pereira, J. et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 23, 1127–1139 (2016).
de Picciotto, N. E. et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530 (2016).
Gomes, A. P. et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155, 1624–1638 (2013).
Guan, Y. et al. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1-dependent manner. J. Am. Soc. Nephrol. 28, 2337–2352 (2017).
Li, J. et al. A conserved NAD+ binding pocket that regulates protein–protein interactions during aging. Science 355, 1312–1317 (2017).
Mouchiroud, L. et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441 (2013).
Scheibye-Knudsen, M. et al. A high-fat diet and NAD+ activate Sirt1 to rescue premature aging in Cockayne syndrome. Cell Metab. 20, 840–855 (2014).
Tarrago, M. G. et al. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD+ decline. Cell Metab. 27, 1081–1095 (2018).
Williams, P. A. et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355, 756–760 (2017).
Yoshino, J., Mills, K. F., Yoon, M. J. & Imai, S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536 (2011).
Stein, L. R. & Imai, S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 33, 1321–1340 (2014).
Johnson, S., Wozniak, D. F. & Imai, S. CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. NPG Aging Mech. Dis. 4, 10 (2018).
Yoshida, M. et al. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 30, 329–342 (2019).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69, S4–S9 (2014).
Andriani, G. A. et al. Whole-chromosome instability induces senescence and promotes SASP. Sci. Rep. 6, 35218 (2016).
Rodier, F. et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 11, 973–979 (2009).
Wiley, C. D. et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 23, 303–314 (2016).
Malavasi, F. et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 88, 841–886 (2008).
Chini, C. et al. The NADase CD38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular NAD+ decline. Biochem. Biophys. Res. Commun. 513, 486–493 (2019).
da Silva, C. P. et al. Ectocellular CD38-catalyzed synthesis and intracellular Ca2+-signalling activity of cyclic ADP-ribose in T-lymphocytes are not functionally related. FEBS Lett. 439, 291–296 (1998).
Horenstein, A. L. et al. NAD+-metabolizing ectoenzymes in remodeling tumor–host interactions: the human myeloma model. Cells 4, 520–537 (2015).
Horenstein, A. L. et al. Adenosine generated in the bone marrow niche through a CD38-mediated pathway correlates with progression of human myeloma. Mol. Med 22, 694–704 (2016).
van de Donk, N., Richardson, P. G. & Malavasi, F. CD38 antibodies in multiple myeloma: back to the future. Blood 131, 13–29 (2018).
Sun, L. et al. A novel mechanism for coupling cellular intermediary metabolism to cytosolic Ca2+ signaling via CD38/ADP-ribosyl cyclase, a putative intracellular NAD+ sensor. FASEB J. 16, 302–314 (2002).
Mottahedeh, J. et al. CD38 is methylated in prostate cancer and regulates extracellular NAD. Cancer Metab. 6, 13 (2018).
Aksoy, P. et al. Regulation of SIRT 1-mediated NAD-dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem. Biophys. Res. Commun. 349, 353–359 (2006).
Aksoy, P., White, T. A., Thompson, M. & Chini, E. N. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem. Biophys. Res. Commun. 345, 1386–1392 (2006).
Liang, M., Chini, E. N., Cheng, J. & Dousa, T. P. Synthesis of NAADP and cADPR in mitochondria. Arch. Biochem. Biophys. 371, 317–325 (1999).
Zhao, Y. J., Lam, C. M. & Lee, H. C. The membrane-bound enzyme CD38 exists in two opposing orientations. Sci. Signal. 5, ra67 (2012).
Funaro, A. et al. Identification and characterization of an active soluble form of human CD38 in normal and pathological fluids. Int Immunol. 8, 1643–1650 (1996).
Zielinska, W., Barata, H. & Chini, E. N. Metabolism of cyclic ADP-ribose: zinc is an endogenous modulator of the cyclase/NAD glycohydrolase ratio of a CD38-like enzyme from human seminal fluid. Life Sci. 74, 1781–1790 (2004).
De Flora, A., Guida, L., Franco, L. & Zocchi, E. The CD38/cyclic ADP-ribose system: a topological paradox. Int. J. Biochem. Cell Biol. 29, 1149–1166 (1997).
Boslett, J., Helal, M., Chini, E. & Zweier, J. L. Genetic deletion of CD38 confers post-ischemic myocardial protection through preserved pyridine nucleotides. J. Mol. Cell Cardiol. 118, 81–94 (2018).
Chatterjee, S. et al. CD38-NAD+ axis regulates immunotherapeutic anti-tumor T cell response. Cell Metab. 27, 85–100 (2018).
Lee, C. U., Song, E. K., Yoo, C. H., Kwak, Y. K. & Han, M. K. Lipopolysaccharide induces CD38 expression and solubilization in J774 macrophage cells. Mol. Cells 34, 573–576 (2012).
Matalonga, J. et al. The nuclear receptor LXR limits bacterial infection of host macrophages through a mechanism that impacts cellular NAD metabolism. Cell Rep. 18, 1241–1255 (2017).
Partida-Sanchez, S. et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 7, 1209–1216 (2001).
Jablonski, K. A. et al. Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE 10, e0145342 (2015).
Baker, D. J. et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays aging-associated disorders. Nature 479, 232–236 (2011).
Biran, A. et al. Quantitative identification of senescent cells in aging and disease. Aging Cell 16, 661–671 (2017).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Xu, M. et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl Acad. Sci. USA 112, E6301–E6310 (2015).
De Cecco, M. et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566, 73–78 (2019).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
Billington, R. A. et al. Emerging functions of extracellular pyridine nucleotides. Mol. Med 12, 324–327 (2006).
Deckert, J. et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin. Cancer Res. 20, 4574–4583 (2014).
Becherer, J. D. et al. Discovery of 4-amino-8-quinoline carboxamides as novel, submicromolar inhibitors of NAD-hydrolyzing enzyme CD38. J. Med. Chem. 58, 7021–7056 (2015).
Grozio, A. et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat. Metab. 1, 47–57 (2019).
Minhas, P. S. et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat. Immunol. 20, 50–63 (2019).
Preugschat, F. et al. A pre-steady state and steady state kinetic analysis of the N-ribosyl hydrolase activity of hCD157. Arch. Biochem. Biophys. 564, 156–163 (2014).
Liu, L. et al. Lipopolysaccharide activates ERK–PARP-1–RelA pathway and promotes nuclear factor–κB transcription in murine macrophages. Hum. Immunol. 73, 439–447 (2012).
Liu, L. et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 27, 1067–1080 (2018). e1065.
Lumeng, C. N. et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J. Immunol. 187, 6208–6216 (2011).
Singh, P. et al. Lymphoid neogenesis and immune infiltration in aged liver. Hepatology 47, 1680–1690 (2008).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Ghosh, S. et al. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J. Gerontol. A Biol. Sci. Med. Sci. 70, 232–246 (2015).
Kim, K. A., Jeong, J. J., Yoo, S. Y. & Kim, D. H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 16, 9 (2016).
Grozio, A. et al. CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J. Biol. Chem. 288, 25938–25949 (2013).
Covarrubias, A. J. et al. Senescent cells promote tissue NAD+ decline during aging via the activation of CD38+ macrophages. Nat. Metab. https://doi.org/10.1038/s42255-020-00305-3 (2020).
Maier, B. et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 18, 306–319 (2004).
Detalle, L. et al. Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrob. Agents Chemother. 60, 6–13 (2016).
Enever, C., Batuwangala, T., Plummer, C. & Sepp, A. Next generation immunotherapeutics—honing the magic bullet. Curr. Opin. Biotechnol. 20, 405–411 (2009).
Hamers-Casterman, C. et al. Naturally occurring antibodies devoid of light chains. Nature 363, 446–448 (1993).
Konning, D. et al. Camelid and shark single domain antibodies: structural features and therapeutic potential. Curr. Opin. Struct. Biol. 45, 10–16 (2017).
Holliger, P. & Hudson, P. J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 23, 1126–1136 (2005).
Lauwereys, M. et al. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 17, 3512–3520 (1998).
Wesolowski, J. et al. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med. Microbiol. Immunol. 198, 157–174 (2009).
Yu, Y. et al. Humanized CD7 nanobody-based immunotoxins exhibit promising anti-T-cell acute lymphoblastic leukemia potential. Int. J. Nanomed. 12, 1969–1983 (2017).
Harris, K. E. et al. Sequence-based discovery demonstrates that fixed light chain human transgenic rats produce a diverse repertoire of antigen-specific antibodies. Front. Immunol. 9, 889 (2018).
Osborn, M. J. et al. High-affinity IgG antibodies develop naturally in Ig-knockout rats carrying germline human IgH/Igκ/Igλ loci bearing the rat CH region. J. Immunol. 190, 1481–1490 (2013).
Vafa, O. et al. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods 65, 114–126 (2014).
Yoshino, J. & Imai, S. Accurate measurement of NAD+ with high-performance liquid chromatography. Methods Mol. Biol. 1077, 203–215 (2013).
Cartwright, M. J. et al. Aging, depot origin, and preadipocyte gene expression. J. Gerontol. A Biol. Sci. Med. Sci. 65, 242–251 (2010).
Babu, J. R. et al. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J. Cell Biol. 160, 341–353 (2003).
Acknowledgements
This work was supported in part by grants from the Helen Diller Family Foundation, Ted Nash Long Life Foundation, the Glenn Foundation for Medical Research via the Paul F. Glenn Laboratories for the Biology of Aging at the Mayo Clinic (E.N.C, J.M.v.D. and D.B), sponsored research funding from Calico Life Sciences, the Mayo and Noaber Foundations, NIH National Institute of Aging (NIA) grants AG-26094 (to E.N.C.), AG58812 (to E.N.C.), CA233790 (to E.N.C.), AG13925 (to J.L.K.), AG057493 (to J.M.v.D.), AG016694 (to J.M.S.) and P01 AG051449 (to J.M.S). This work was also supported by grants from the National Institute of Diabetes, Digestive and Kidney disease (DK098656 to J.A.B.) R.J. Ryan and T.W. Ryan (to J.L.K.) and the Connor Group (to J.L.K.). A.S.P. thanks the Colton Center for Auto-Immunity at NYU Langone for funding support.
Author information
Authors and Affiliations
Contributions
E.N.C. and C.C.S.C. generated the original hypothesis and concept of the manuscript. All authors contributed to the development of the hypothesis and experimental approaches. C.C.S.C., T.R.P. and K.A.H. performed cell isolations and experiments. M.G.T., G.C.d.O., J.M.E.N, T.R.P., S.K., K.T., G.A., J.M.S. and M.D.C. designed and performed animal studies. B.G.C., D.J.B. and J.M.v.D. established the INK-ATTAC cohort, treated mice with AP or vehicle and prepared tissues for expression analyses. J.K. and T.T. contributed with reagents and design for senescence experiments. C.C.S.C., E.N.C., M.G.T., T.R.P., J.M.E.N., K.S.K., G.C.d.O., L.S.G., G.A., M.A.W., P.V., A.L.K., K.C., M.R.M., C.J., J.D.R. and J.A.B. designed, performed and interpreted enzymatic analyses and nucleotide measurements. A.S.P. and S.K. performed flow cytometry. S.K. and G.M.W. performed immunohistochemistry. K.A.H., G.M.W. and L.S.G. performed PCR analyses. K.A.H. performed cytokine measurements. C.C.S.C., M.A.W. and G.M.W. made CD38 constructs. W.v.S., K.D. and S.C. developed and screened the anti-CD38 antibodies. J.M.E.N. developed the method used to measure NMN in the serum. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
E.N.C. holds a patent on the use of CD38 inhibitors for metabolic diseases that is licensed by Elysium health. E.N.C. is a consultant for TeneoBio, Calico, Mitobridge and Cytokinetics. E.N.C. is on the advisory board of Eolo Pharma. W.v.S. is the Chief Scientific Officer of Teneobio, a company interested in the development of therapeutic antibodies. W.v.S., K.D., E.N.C. and S.C. own stocks in Teneobio. J.L.K., T.T., J.M.v.D. and D.J.B. have a financial interest related to this research: patents on transgenic animals capable of being induced to delete senescent cells are held by the Mayo Clinic. J.L.K., T.T., D.J.B. and J.M.v.D. are co-inventors on patent applications licensed to or filed by Unity Biotechnology, a company developing senolytic medicines, including small molecules that selectively eliminate senescent cells. J.M.v.D. is a co-founder of Unity Biotechnology. J.M.S. is a co-founder of Transposon Therapeutics, serves as chair of its Scientific Advisory Board and consults for Astellas Innovation Management, Atropos Therapeutics and Gilead Sciences. J.D.R. is a member of the Rutgers Cancer Institute of New Jersey and of the University of Pennsylvania Diabetes Research Center; a co-founder and stockholder in VL54, Sofro and Raze Therapeutics; and advisor and stockholder in Agios Pharmaceuticals, Kadmon Pharmaceuticals, Bantam Pharmaceuticals, Colorado Research Partners, Rafael Pharmaceuticals and L.E.A.F. Pharmaceuticals. J.B. has intellectual property related to the use of NAD precursors in liver regeneration. All other authors declare no conflict of interest. This research was conducted in compliance with Mayo Clinic Conflict of Interest policies.
Additional information
Peer review information Primary Handling Editor: Pooja Jha.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 CD38+ cells increase in tissues with aging.
a, Graphs show no change in CD38+CD31+ cell population in WAT and liver tissues between young (4 month-old) and old (32 month-old) mice (n=3 for liver and n=9 mice for WAT). b, Gating strategy used to show the CD45+CD38+ population in WAT of young (4 month-old) and old (22 month-old) mice. c, Histograms of CD38+ population in young (4 month-old) and old (22 month-old) WAT showing the shift in CD38 expression. The graph shows the relative area under the curve (AUC) for young and old (n=5 mice per group). d, Histograms showing the CD38 expression in different immune cells in young (4 month-old) and old (32 month-old) liver. Table shows cell counts for each subset of immune cells, the data are representative of n=5 mice per group. Data are mean ± SEM, analyzed by unpaired two-sided t-test.
Extended Data Fig. 2 CD38 increases in tissues after LPS administration, bone marrow transplant of WT cells into CD38 KO mice, and in activated macrophages.
a, b, 12 month-old mice received daily subcutaneous injection of vehicle (Ctrl) or LPS (300 μg/kg) for 5 days (n=9 mice per group). (a) mRNA expression of Cd38, F4/80, and Cd45 in subcutaneous WAT (Subq WAT) measured by qRT-PCR analysis and expressed relative to Ctrl. (b) Immunofluorescent staining for CD38 (red) and CD45 (green) in Subq WAT, showing accumulation of CD38+CD45+ cells with LPS treatment. Images are representative of 6 mice per group. c-h, Sub-lethally irradiated CD38 KO mice were subjected to bone marrow transplant (BMT) with 1 × 106 bone marrow cells (BMC) per animal from either WT (WT>KO) or CD38 KO donors (KO>KO). Twelve weeks after transplantation, mice were subcutaneously injected with LPS (300 μg/kg) or vehicle daily for 5 days and harvested at day 5. (c,d) CD38 activity and NAD+ levels in spleen and jejunum (n=4 mice per group). (e) CD38 expression by immunoblot (n=3 mice per group). (f) Number of CD38+ cells by flow cytometry was measured in spleen and jejunum with and without LPS treatment (n=5 mice per group). (g,h) CD38 activity and NAD+ levels in liver and pancreas (n=4 mice per group). i, CD38 activity was measured in macrophages isolated from WT or CD38 KO mice and treated or not with LPS (100 ng/mL) with and without the CD38 inhibitor 78c (0.5 μM) for 24 hours. CD38 activity is relative to Ctrl WT (n=3 biologically independent samples). j, Macrophages isolated from WT or CD38 KO mice were treated or not with LPS (100 ng/mL) for 24 hours and mRNA expression of Cd38 and other markers of macrophage activation were measured. Expression was measured by qRT-PCR analysis and expressed relative to Ctrl WT (n=4 biologically independent samples). Data are mean ± SEM, analyzed by unpaired two-sided t-test, NS=non-significant.
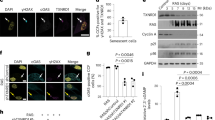
Extended Data Fig. 3 Senescence regulates CD38 in vivo.
a, WT macrophages were incubated with LPS (100 ng/mL) in the presence or absence of 100 nM AP20187 for 20 hours. CD38 activity was measured in cell lysates (n=4 biologically independent samples). b, WT macrophages were treated with LPS (100 ng/mL) for 20 hours and cell lysates were prepared. Lysates were incubated with or without AP20187 for 15 min before CD38 activity was measured (n=3 biologically independent samples). c-g, 12 month-old INK-ATTAC mice were treated with vehicle or with AP20187 for 16 months (28 month-old groups) and were compared with 12 month-old animals. (c) Representative images of immunofluorescent staining for CD38 (red), ORF1 (yellow), and CD45 (green) in WAT. Insets show the image of 28 month-old WAT with removal of green color channel CD45 signal (left) and with removal of red color channel CD38 signal (right) to show accumulation of CD38+ CD45+ cells near ORF1+ cells. Graph shows quantification of CD45+ immune clusters in WAT, based on 20 5x fields per sample (12 mo, n=5; 28 mo, n=4; 28 mo+AP, n=3 mice). (d) IL6 levels in WAT detected by ELISA (12 mo n=5; 28 mo n=7; 28 mo+AP n=6 mice). (e) NMN levels measured in WAT (12 mo n=5; 28 mo n=6; 28 mo+AP n=13 mice). (f) Relative mRNA levels of p16 (12 mo n=6; 28 mo n=7; 28 mo+AP n=5 mice) and Cd38 (12 mo n=10; 28 mo n=9; 28 mo+AP n=6 mice) detected by qRT-PCR analysis in liver. Levels are relative to 12 month-old mice. (g) NAD+ levels in liver (12 mo n=6; 28 mo n=7; 28 mo+AP n=15 mice). h, Relative mRNA levels of inflammatory and senescence-related genes in X-ray irradiated WT mouse pre-adipocytes determined by qRT-PCR. Levels are relative to control non-senescent cells (Ctrl) (Ctrl n=5; X-ray n=6 biologically independent samples). i, Quantitative analysis of cytokines/chemokines in conditioned media harvested from irradiated (CM-SEN) or non-senescent (CM-NS) mouse pre-adipocytes. Heat maps reflect analytes (pg/mL) measured in 3–25-plex Luminex assays. Heat map shows average of 5 biologically independent samples. Data are mean ± SEM, except letters e-g that are mean ± SD, analyzed by unpaired two-sided t-test.
Extended Data Fig. 4 The senescence-associated secretory phenotype (SASP) increases CD38 accumulation.
a, b, WT macrophages were incubated with conditioned media from senescent (CM-SEN) and non-senescent (CM-NS) mouse pre-adipocytes for 20 hours. CD38 expression and activity are relative to CM-NS. (a) Cd38 mRNA expression in the macrophages was measured by qRT-PCR analysis. (n=5 biologically independent samples). (b) Relative CD38 activity in macrophage lysates (n=5 biologically independent samples). c, WT macrophages were treated with LPS (100 ng/mL) with and without 3TC (10 μM) for 20 hours and CD38 activity was measured in cell lysates (n=3 biologically independent samples). d, WT macrophages were incubated with conditioned media from senescent (CM-SEN) and non-senescent (CM-NS) mouse pre-adipocytes for 20 hours. Conditioned media was pre-incubated for 2 hours with or without IL6 or TNF-α antibody (5 μg/mL) before addition to the macrophages. CD38 activity was measured in cell lysates, and expressed relative to CM-NS (CM-NS and CM-SEN n=8; CM-NS+IL6 Ab and CM-SEN+IL6 n=6; CM-NS+TNF Ab and CM-SEN+TNF n=4 biologically independent samples). e, HUVECs were treated with conditioned media from senescent (CM-SEN) and non-senescent (CM-NS) mouse embryonic fibroblasts for 20 hours. CM was pre-incubated for 2 hours with or without TNF-α antibody (5 μg/mL) before addition to the HUVECs. Cd38 mRNA expression was measured by qRT-PCR analysis (CM-NS n=12; CM-NS+TNF Ab n=4; CM-SEN n=10; CM-SEN+TNF Ab n=4 biologically independent samples). Data are mean ± SEM, analyzed by unpaired two-sided t-test.
Extended Data Fig. 5 CD38 is required for genotoxic/senescence-induced NAD+ decline.
a, Cd68 mRNA expression measured by qRT-PCR (n=5 mice per group, except for CI Ctrl where n=4 mice). Data are mean ± SEM, analyzed by unpaired two-sided t-test.
Extended Data Fig. 6 Characterization of the inhibitory CD38 antibody in human cells.
a, 293T cells were transfected with vector (V), CD38 WT (WT), CD38 CI (CI) or CD38 Δ49 (Δ49). Representative immunoblotting of 4 independent experiments showing CD38 expression in cytosol of 293T cells transfected with CD38 plasmids. b, Immunofluorescence of fixed 293T cells transfected with CD38 plasmids. Images are representative of 3 independent experiments. Cells were labeled with CD38 antibody and a membrane dye, followed by Hoechst staining. Images show separate CD38 and membrane staining. c, d, Lysates of transfected 293T cells were incubated with varied concentrations of the human CD38 antibody isatuximab (isa) or 0.5 μM 78c for 15 min before CD38 activity was measured. Activity is relative to control (no antibody). (c) Lysates of 293T transfected with CD38 WT plasmid (n=4 biologically independent samples). (d) Lysates of 293T transfected with CD38 Δ49 plasmid (n=3 biologically independent samples). e, Human recombinant CD38 (hCD38) (100 ng/mL) was incubated with and without isatuximab (5 μg/mL) for 2 hours and then added to 293T cells together with NMN (300 μM). Control samples had no CD38 and no NMN. 293T were incubated with NMN and hCD38 for 20 hours and NAD+ levels were measured in the 293T cells. Values show the difference from untreated control (n=3 except for NMN+hCD38+isa where n=4 biologically independent samples). f, Scheme representing the coculture model. g, NAD+ levels in AML 12 cells co-cultured with 293T cells expressing vector or CD38 WT. 293T cells were treated with and without 200 μM NMN 20 hours after transfection in the presence or absence of 5 μg/ml isatuximab. AML 12 cells were collected 20 hours after incubation with 293T cells (n=3 except for V+NMN where n=4 biologically independent samples). Levels are relative to vector-transfected cells. Data are mean ± SEM, analyzed by unpaired two-sided t-test.
Extended Data Fig. 7 Characterization of the mouse CD38 antibody.
a, Relative mRNA expression of Nampt and Cd157 after treatment of BMDMs of WT and CD38 KO mice with and without 100 ng/mL LPS for 24 hours. Expression, assessed by qRT-PCR, is relative to Ctrl WT (n=4 biologically independent samples). b–d, Characterization of the mouse anti-CD38 antibodies used in this study. b, Protein and cell-based screens to discover anti-CD38 UniAbs. c, Graph showing the binding of FITC-labeled Ab68 to freshly isolated mouse spleen cells (n=3 biologically independent samples). d, Effect of Ab68 on the rate of CD38 hydrolase activity at different concentrations of substrate (left, n=8; except for no e-NAD where n=4 biologically independent samples and respective Lineweaver-Burk plots (right, n=6 biologically independent samples).
Extended Data Fig. 8 Characterization of the ecto-enzymatic activity of CD38 in BMDMs.
a, Table shows the binding affinities (KD) of anti-mouse CD38 antibodies Ab68 and Ab69 and a mouse control antibody OKT3. NB=No Binding. b, Apoptosis of CHO cells stably transfected with mouse CD38 after incubation with antibodies Ab68, Ab69, and NIMR-5 for 24 hours (n=2 samples per concentration). c, Cell viability of WT macrophages treated with LPS (100 ng/mL), with or without Ab68 or Ab69 (5 g/mL) for 24 hours (n=4 except for LPS+Ab69 where n=3 biologically independent samples). d, Graph shows the internalization of Ab68 compared to control antibody NIMR-5 after 45 minutes and 1.5 hours. e, NAD+ levels in AML 12 treated with 200 M NMN in the presence or absence of LPS (100 ng/mL), Ab68 (5 g/mL), and 78c (0.5 M). LPS was given for 18 hours, then Ab68 was added, and 3 hours later NMN was added. Cells were collected 20 hours after NMN was added. NAD+ levels were relative to control (Ctrl) (n=4 biologically independent samples). f, NAD+ levels in AML 12 cells cocultured with macrophages from CD38 KO mice. LPS was given for 18 hours to the macrophages, then NMN or NR (200 M) were added for 3 hours. Next, macrophages were incubated with AMLs, and AML cells were collected 20 hours later. NAD+ levels were relative to control (Ctrl) (n=4 biologically independent samples). g, NAD+ levels in AML 12 cocultured with macrophages. Macrophages were treated with 200 M NA in the presence or absence of LPS (100 ng/mL), and Ab68 (5 g/mL). LPS was given for 18 hours, then Ab68 was added, and 3 hours later nicotinic acid (NA) was added to the macrophages.Three hours after addition of NA, macrophages were incubated with AML cells. AML cells were collected 20 hours later and NAD+ levels were calculated relative to control (Ctrl) (n=5 biologically independent samples). h, Macrophages were incubated with conditioned media from senescent (CM-SEN) andnon-senescent (CM-NS) mouse pre-adipocytes, and with LPS and without (Ctrl) for 20 hours. Cd38 mRNA expression (CM-NS and CM-SEN n=5; Ctrl and LPS n=4 biologically independent samples), CD38 activity (n=5 biologically independent samples), and NAD+ levels (n=6 biologically independent samples) were measured in the macrophages. Samples from cells incubated with CM-SEN, were calculated relative to cells incubated with CM-NS. Samples from cells treated with LPS, were calculated relative to vehicle-treated cells (Ctrl). Data are mean ± SEM, analyzed by unpaired two-sided t-test, NS= non-significant.
Extended Data Fig. 9 Blocking the CD38 ecto-enzymatic activity regulates NMN, NAD+ levels and CD38 activity in vivo.
a,b, 4 month-old WT and CD38 KO mice were treated with vehicle (Ctrl), Ab68 (5 mg/kg), or Ab69 (5 mg/kg) on day 1 and day 5, and euthanized on day 8. a, CD38 activity in several tissues (liver n=5 except for Ctrl CD38 KO where n=4 mice; skeletal muscle n=3 except for Ab68 where n=2 mice; jejunum and spleen n=5 mice per group). b, NAD+ levels in several tissues (liver and spleen n=5 mice except for Ctrl CD38 KO where n=4; skeletal muscle and spleen n=5 mice per group). c, 4 month-old WT mice were treated with a single injection of vehicle (Ctrl) or Ab68 (5 mg/kg). After 3 days, tissues were harvested and gene expression was measured in WAT byqRT-PCR. Values are relative to Ctrl (Ctrl n=5 mice; Ab68 n=4 mice). d, 4 month-old WT mice were treated with vehicle (Ctrl) or Ab68 (5 mg/kg) on day 1and day 5, and euthanized on day 8. NMN and nicotinamide (NAM) levels were measured in liver and skeletal muscle. Levels are relative to Ctrl (n=5 miceper group). e, Relative NMN levels in WAT, liver and skeletal muscle of 4 month-old WT and CD38 KO mice. Levels are relative to WT mice (n=5 mice per group except for liver CD38 KO where n=3 mice and skeletal muscle CD38 KO where n=4 mice).
Extended Data Fig. 10 Blocking the CD38 ecto-enzymatic activity regulates NMN and NAD+ levels.
a, 22 month-old WT mice were injected with a single dose of vehicle (Ctrl) or Ab68 (5 mg/kg). Mice were euthanized at different time points, and NMN and NAD levels were measured in liver. Graphs show. the time course of the relative increase of NMN or NAD+ in vehicle and Ab68-treated over levels at time 0 (n=4 mice per group). b, Graph shows the difference between NMNase activity of CD38 in the blood of WT and CD38 KO mice (n=3 mice per group). c, Human recombinant CD38 enzyme was incubated withNMN at different pHs, and levels of nicotinamide were measured by an enzymatic coupled reaction (n=3 biologically independent samples). d, Relative levels of NAD+ and NR in the serum of mice injected with a single injection of vehicle (Ctrl) or Ab68 (5 mg/kg). Mice were euthanized 3 days later and NAD and NR levels in the serum were measured (n=6 mice per group). e, Relative mRNA expression of inflammatory and SASP markers in WAT of 3 month-old (young) and18 month-old mice (old) treated or not with a single injection of vehicle (Ctrl) or 5 mg/kg Ab68. WAT was collected 3 days after injection of Ab68 (n=5 except for Ctrl old where n=4). f, Schematic representation of the role of senescence and sterile inflammation in regulation of CD38 in immune cells, and the effect of extracellular NMN degradation in the regulation of tissue NAD+ levels. The senescence-associated secretory phenotype (SASP) increases the expression/accumulation of CD38 in immune cells in tissues where immune cells appear to regulate tissue NAD levels. It has been proposed that NMN enters the cell through a putative NMN transporter, is converted to NAD+ intracellularly, and increases intracellular NAD+ levels (1). In the presence of senescence cells and the sterile inflammation, the levels of the ectoenzyme CD38 appear to increase in immune cells and tissues, degrading extracellular NMN to NAM, and causing a decrease in NMN/NAD+ levels in tissues (2). Data are mean ± SEM, analyzed by unpaired two-sided t-test, except for (a,b) where data are analyzed by two-way ANOVA.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Supplementary Table 1
TaqMan probes used in real-time qPCR assays.
Source data
Source Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 6
Unprocessed western blots and/or gels.
Rights and permissions
About this article
Cite this article
Chini, C.C.S., Peclat, T.R., Warner, G.M. et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat Metab 2, 1284–1304 (2020). https://doi.org/10.1038/s42255-020-00298-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-00298-z
This article is cited by
-
Absolute quantification of nicotinamide mononucleotide in biological samples by double isotope-mediated liquid chromatography-tandem mass spectrometry (dimeLC-MS/MS)
npj Aging (2024)
-
Targeting NAD Metabolism for the Therapy of Age-Related Neurodegenerative Diseases
Neuroscience Bulletin (2024)
-
Human skeletal muscle aging atlas
Nature Aging (2024)
-
NAD+ metabolism-based immunoregulation and therapeutic potential
Cell & Bioscience (2023)
-
Impact of NAD+ metabolism on ovarian aging
Immunity & Ageing (2023)