Abstract
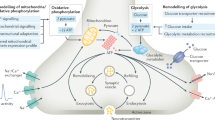
Mitochondria supply ATP essential for synaptic transmission. Neurons face exceptional challenges in maintaining energy homoeostasis at synapses. Regulation of mitochondrial trafficking and anchoring is critical for neurons to meet increased energy consumption during sustained synaptic activity. However, mechanisms recruiting and retaining presynaptic mitochondria in sensing synaptic ATP levels remain elusive. Here we reveal an energy signalling axis that controls presynaptic mitochondrial maintenance. Activity-induced presynaptic energy deficits can be rescued by recruiting mitochondria through the AMP-activated protein kinase (AMPK)–p21-activated kinase (PAK) energy signalling pathway. Synaptic activity induces AMPK activation within axonal compartments and AMPK–PAK signalling triggers phosphorylation of myosin VI, which drives mitochondrial recruitment and syntaphilin-mediated anchoring on presynaptic filamentous actin. This pathway maintains presynaptic energy supply and calcium clearance during intensive synaptic activity. Disrupting this signalling cross-talk triggers local energy deficits and intracellular calcium build-up, leading to impaired synaptic efficacy during trains of stimulation and reduced recovery from synaptic depression after prolonged synaptic activity. Our study reveals a mechanistic cross-talk between energy sensing and mitochondria anchoring to maintain presynaptic metabolism, thus fine-tuning short-term synaptic plasticity and prolonged synaptic efficacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author. The raw datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Harris, J. J., Jolivet, R. & Attwell, D. Synaptic energy use and supply. Neuron 75, 762–777 (2012).
Sheng, Z. H. The interplay of axonal energy homeostasis and mitochondrial trafficking and anchoring. Trends Cell Biol. 27, 403–416 (2017).
Devine, M. J. & Kittler, J. T. Mitochondria at the neuronal presynapse in health and disease. Nat. Rev. Neurosci. 19, 63–80 (2018).
Sun, T., Qiao, H., Pan, P. Y., Chen, Y. & Sheng, Z. H. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 4, 413–419 (2013).
Ashrafi, G. & Ryan, T. A. Glucose metabolism in nerve terminals. Curr. Opin. Neurobiol. 45, 156–161 (2017).
Hubley, M. J., Locke, B. R. & Moerland, T. S. The effects of temperature, pH and magnesium on the diffusion coefficient of ATP in solutions of physiological ionic strength. Biochim. Biophys. Acta 1291, 115–121 (1996).
Verstreken, P. et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47, 365–378 (2005).
Smith, H. L. et al. Mitochondrial support of persistent presynaptic vesicle mobilization with age-dependent synaptic growth after LTP. eLife 5, e15275 (2016).
Pathak, D., Berthet, A. & Nakamura, K. Energy failure—does it contribute to neurodegeneration? Ann. Neurol. 74, 506–516 (2013).
Cingolani, L. A. & Goda, Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 9, 344–356 (2008).
Morris, R. L. & Hollenbeck, P. J. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J. Cell Biol. 131, 1315–1326 (1995).
Chada, S. R. & Hollenbeck, P. J. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr. Biol. 14, 1272–1276 (2004).
Hirokawa, N. & Takemura, R. Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 6, 201–214 (2005).
Quintero, O. A. et al. Human Myo19 is a novel myosin that associates with mitochondria. Curr. Biol. 19, 2008–2013 (2009).
Wang, X. & Schwarz, T. L. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 136, 163–174 (2009).
MacAskill, A. F. et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61, 541–555 (2009).
Sheng, Z. H. Mitochondrial trafficking and anchoring in neurons: new insight and implications. J. Cell Biol. 204, 1087–1098 (2014).
Kang, J. S. et al. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell 132, 137–148 (2008).
Zhou, B. et al. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J. Cell Biol. 214, 103–119 (2016).
Chen, Y. & Sheng, Z. H. Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport. J. Cell Biol. 202, 351–364 (2013).
Gutnick, A., Banghart, M. R., West, E. R. & Schwarz, T. L. The light-sensitive dimerizer zapalog reveals distinct modes of immobilization for axonal mitochondria. Nat. Cell Biol. 21, 768–777 (2019).
Xiao, B. et al. Structural basis of AMP binding to mammalian AMP-activated protein kinase. Nature 449, 496–500 (2007).
Bokoch, G. M. Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743–781 (2003).
Meng, J., Meng, Y., Hanna, A., Janus, C. & Jia, Z. Abnormal long-lasting synaptic plasticity and cognition in mice lacking the mental retardation gene Pak3. J. Neurosci. 25, 6641–6650 (2005).
Kong, D. et al. A postsynaptic AMPK→p21-activated kinase pathway drives fasting-induced synaptic plasticity in AgRP. Neuron 91, 25–33 (2016).
Pathak, D. et al. The role of mitochondrially derived ATP in synaptic vesicle recycling. J. Biol. Chem. 290, 22325–22336 (2015).
Nakano, M., Imamura, H., Nagai, T. & Noji, H. Ca2+ regulation of mitochondrial ATP synthesis visualized at the single-cell level. ACS Chem. Biol. 6, 709–715 (2011).
Vaccaro, V., Devine, M. J., Higgs, N. F. & Kittler, J. T. Miro1-dependent mitochondrial positioning drives the rescaling of presynaptic Ca2+ signals during homeostatic plasticity. EMBO Rep. 18, 231–240 (2017).
Yu, D. F. et al. HFS-triggered AMPK activation phosphorylates GSK3β and induces E-LTP in rat hippocampus in vivo. CNS Neurosci. Ther. 22, 525–531 (2016).
Marinageli, C. et al. AMP-activated protein kinase is essential for the maintenance of energy levels during synaptic activation. iScience 9, 1–13 (2018).
Konagaya, Y. et al. A highly sensitive FRET biosensor for AMPK exhibits heterogeneous AMPK responses among cells and organs. Cell Rep. 21, 2628–2638 (2017).
Hurley, R. L. et al. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280, 29060–29066 (2005).
Woods, A. et al. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2, 21–33 (2005).
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135 (2018).
Corton, J. M., Gillespie, J. G., Hawley, S. A. & Hardie, D. G. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 229, 558–565 (1995).
Hamann, J. C. et al. Entosis is induced by glucose starvation. Cell Rep. 20, 201–210 (2017).
Sheng, Z. H. & Cai, Q. Mitochondrial transport in neurons impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 13, 77–93 (2012).
Coles, C. H. & Bradke, F. Coordinating neuronal actin–microtubule dynamics. Curr. Biol. 25, R677–R691 (2015).
Osterweil, E., Wells, D. G. & Mooseker, M. S. A role for myosin VI in postsynaptic strcture and glutamate receptor endocytosis. J. Cell Biol. 168, 329–338 (2005).
Yano, H. et al. BDNF-mediated neurotransmission relies upon a myosin VI motor complex. Nat. Neurosci. 9, 1009–1018 (2006).
Pathak, D., Sepp, K. J. & Hollenbeck, P. J. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J. Neurosci. 30, 8984–8992 (2010).
Morton, W. M., Ayscough, K. R. & McLaughlin, P. J. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2, 376–378 (2000).
Tumbarello, D. A., Kendrick-Jones, J. & Buss, F. Myosin VI and its cargo adaptors—linking endocytosis and autophagy. J. Cell Sci. 126, 2561–2570 (2013).
Srivastava, J. & Barber, D. Actin co-sedimentation assay; for the analysis of protein binding to F-actin. J. Vis. Exp. 13, 690 (2008).
Sjoblom, B., Salmazo, A. & Djinovic-Carugo, K. α-Actinin structure and regulation. Cell. Mol. Life Sci. 65, 2688–2701 (2008).
Alam, M. S. Proximity ligation assay (PLA). Curr. Protoc. Immunol. 123, e58 (2018).
Masters, T. A., Tumbarello, D. A., Chibalina, M. V. & Buss, F. MYO6 regulates spatial organization of signaling endosomes driving AKT activation and actin dynamics. Cell Rep. 19, 2088–2101 (2017).
Lin, M. Y. et al. Releasing syntaphilin removes stressed mitochondria from axons independent of mitophagy under pathophysiological conditions. Neuron 94, 595–610(2017).
Spudich, G. et al. Myosin VI targeting to clathrin-coated structures and dimerization is mediated by binding to Disabled-2 and PtdIns(4,5)P2. Nat. Cell Biol. 9, 176–183 (2007).
Thevenot, E. et al. p21-activated kinase 3 (PAK3) protein regulates synaptic transmission through its interaction with the Nck2/Grb4 protein adaptor. J. Biol. Chem. 286, 40044–40059 (2011).
Campbell, H. K., Salvi, A. M., O’Brien, T., Superfine, R. & DeMali, K. A. PAK2 links cell survival to mechanotransduction and metabolism. J. Cell Biol. 218, 1958–1971 (2019).
Buss, F. et al. The localization of myosin VI at the golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation. J. Cell Biol. 143, 1535–1545 (1998).
Brooks, A. B. et al. MYO6 is targeted by Salmonella virulence effectors to trigger PI3-kinase signaling and pathogen invasion into host cells. Proc. Natl Acad. Sci. USA 114, 3915–3920 (2017).
Huang, W. et al. p21-activated kinases 1 and 3 control brain size through coordinating neuronal complexity and synaptic properties. Mol. Cell. Biol. 31, 388–403 (2011).
Lei, M. et al. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell 102, 387–397 (2000).
Deacon, S. W. et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 15, 322–331 (2008).
Di Giovanni, J. & Sheng, Z. H. Regulation of synaptic activity by snapin-mediated endolysosomal transport and sorting. EMBO J. 34, 2059–2077 (2015).
Zucker, R. S. & Regehr, W. G. Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002).
Rangaraju, V., Calloway, N. & Ryan, T. A. Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825–835 (2014).
MacAskill, A. F., Atkin, T. A. & Kittler, J. T. Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur. J. Neurosci. 32, 231–240 (2010).
Billups, B. & Forsythe, I. D. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J. Neurosci. 22, 5840–5847 (2002).
López-Doménech et al. Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. EMBO J. 37, 321–336 (2018).
Xu, K., Zhong, G. & Zhuang, X. Actin, spectrin and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456 (2013).
Ganguly, A. et al. A dynamic formin-dependent deep F-actin network in axons. J. Cell Biol. 210, 401–417 (2015).
Kruppa, A. J. et al. Myosin VI-dependent actin cages encapsulate parkin-positive damaged mitochondria. Dev. Cell 44, 484–499 (2018).
Chang, D. T., Honick, A. S. & Reynolds, I. J. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J. Neurosci. 26, 7035–7045 (2006).
Nguyen, T. T. et al. Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc. Natl Acad. Sci. USA 111, E3631–E3640 (2014).
Courchet, J. et al. Terminal axon branching is regulated by the LKB1–NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell 153, 1510–1525 (2013).
Tao, K., Matsuki, N. & Koyama, R. AMP-activated protein kinase mediates activity-dependent axon branching by recruiting mitochondria to axon. Dev. Neurobiol. 74, 557–573 (2014).
Friel, D. D. & Tsien, R. W. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J. Neurosci. 14, 4007–4024 (1994).
Tang, Y. & Zucker, R. S. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron 18, 483–491 (1997).
Virmani, T., Atasoy, D. & Kavalali, E. T. Synaptic vesicle recycling adapts to chronic changes in activity. J. Neurosci. 26, 2197–2206 (2006).
Jonas, E. A. et al. Modulation of synaptic transmission by the BCL-2 family protein BCL-xL. J. Neurosci. 23, 8423–8431 (2003).
Sakaba, T. & Neher, E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron 32, 1119–1131 (2001).
Vingtdeux, V., Davies, P., Dickson, D. W. & Marambaud, P. AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies. Acta Neuropathol. 121, 337–349 (2011).
Pickett, E. K. et al. Region-specific depletion of synaptic mitochondria in the brains of patients with Alzheimer’s disease. Acta Neuropathol. 136, 747–757 (2018).
Chen, Y. M., Gerwin, C. & Sheng, Z. H. Dynein light chain LC8 regulates syntaphilin-mediated mitochondrial docking in axons. J. Neurosci. 29, 9429–9438 (2009).
Acknowledgements
We thank members of the Sheng laboratory for constructive discussion; K.A. Chamberlain and J.C. Roney for critical reading and editing; K. Remmert and J. Hammer (National Heart, Lung and Blood Institute (NHLBI), NIH) for advice on actin co-sedimentation assay; H. Imamura (Kyoto University), R. Youle (NINDS, NIH), J. Hammer (NHLBI, NIH), J. Kendrick-Jones (MRC LMB), F. Buss (University of Cambridge), D.-Y. Jin (The University of Hong Kong), G. Voeltz (University of Colorado), M. Matsuda (Kyoto University) and D. Trono (EPFL) for sharing constructs; and the NINDS EM Facility and Light Imaging Facility. This work was supported by the Intramural Research Program of NINDS, NIH ZIA NS003029 and ZIA NS002946 (Z.-H.S.).
Author information
Authors and Affiliations
Contributions
S.L. designed and performed cell biology and biochemical experiments and analysed data; G.-J.X. designed and performed electrophysiological analyses; N.H. performed biochemical analyses; Z.-H.S. is the senior author who conceived and directed the project; and S.L. and Z.-H.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Pooja Jha.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Activation of AMPK Signaling by PTX-induced Synaptic Activity.
a–c, Representative immunoblots (a) and quantitative analyses (b, c) showing synaptic activity-induced AMPK signaling in a time-dependent manner. Cortical neurons at DIV14 were treated with PTX (100 μM) for 0, 30, 60 or 120 min, followed by harvesting cell lysates for analysis. Equal amounts (5 μg) of cell lysates were sequentially immunoblotted with antibodies against AMPKα-Thr172 (pAMPKα) and ACC-1-Ser79 (pACC-1), total ACC-1 and AMPKα, and GAPDH (a). The relative intensity of pACC-1 or pAMPKα was calibrated with total ACC-1 or AMPKα respectively, and normalized to the time point at 0 min (b, c). d, e, Validation of AMPK WT and kinase-dead (KD) constructs. Cortical neurons were infected with lentiviruses encoding Flag, Flag-AMPK-WT or Flag-AMPK-KD at DIV4-5, treated with PTX (100 μM for 2 hr) at DIV14, followed by harvesting cell lysates for analysis. Equal amounts (5 μg) of cell lysates were sequentially immunoblotted with antibodies against Flag, AMPK, pACC-1, total ACC-1 and GAPDH. The relative intensity of pACC-1 was calibrated with total ACC-1 levels, and normalized to Flag control. Note that expressing AMPK-WT (P = 0.028), but not AMPK-KD (P = 0.7636), activated AMPK signaling. Data were collected from n = 3 (b, c) or n = 4 (e) independent experiments, expressed as mean ± SEM, and analysed by one-way ANOVA with post hoc testing by Dunnett’s multiple comparisons test (b, c) or by two-sided Fisher’s LSD test (e).
Extended Data Fig. 2 PTX and AICAR Do Not Affect Presynaptic Density and Mitochondrial Integrity.
a, b, Representative images (a) and quantitative analyses (b) showing similar presynaptic density along axons following synaptic activation with PTX or AMPK activation with AICAR. Cortical neurons at DIV14 were treated with PTX (100 μM) or AICAR (1 mM) for 2 hr, followed by co-immunostaining of synaptophysin (SYP) and βIII-tubulin. c, d, Representative images (c) and quantitative analyses (d) showing unaffected mitochondrial integrity upon synaptic activation with PTX or AMPK activation with AICAR. Cortical neurons at DIV14 were treated with PTX (100 μM) or AICAR (1 mM) for 2 hr, followed by co-labeling of total mitochondria with mitoTracker Green (20 nM for 20 min) and mitochondria membrane potential with TMRE (50 nM for 20 min). Mitochondrial integrity was assessed by integrated intensity ratio of TMRE (red) vs mitoTracker (green) within individual mitochondria and normalized to the control group. Data were quantified from the total number of neurons indicated within bars, expressed as mean ± SEM, and analyzed by one-way ANOVA with post hoc testing by Dunnett’s multiple comparisons test. Scale bars: 10 μm.
Extended Data Fig. 3 Myo6 Knockdown Does Not Impact Density of Axonal Mitochondria and Presynaptic Terminals.
a, The alignment of mouse and human myo6 sequences that are targeted by mouse myo6-shRNA. Sequence differences are marked by red color. b, Three immunoblot repeats showing effective myo6 knockdown by myo6-shRNA in mouse cortical neurons. Neurons were infected with lentiviruses encoding scramble (scr) or myo6-shRNA (myo), or combined with co-expression of human myo6 (h-myo) at DIV5 and analyzed by immunoblotting at DIV14. Note that myo6-shRNA effectively depletes myo6 expression in mouse neurons while human myo6 (h-myo) is resistant to mouse myo6-shRNA. c, d, Representative images (c) and quantitative analysis (d) showing effective knockdown of myo6 in mouse cortical neurons. Neurons were infected with lentiviruses encoding scr- or myo6-shRNA at DIV5, followed by co-immunostaining of myo6 and βIII-tubulin at DIV14. e, f, Quantitative analyses revealing no detectable effect of myo6 knockdown on the average density and size of axonal mitochondria (e) and density of presynaptic terminals (f). Cortical neurons at DIV4 were infected lentiviruses encoding with scr-shRNA or myo6-shRNA, followed by co-immunostaining of SV2 and TOM20 at DIV14. The number (per 100 μm) and size (μm2) of axonal mitochondria and the number of presynaptic boutons (per 100 μm) were quantified. g, h, Representative images (g) and quantitative analysis (h) showing no additive reduction in presynaptic mitochondria by myo6 knockdown in snph KO neurons. Neurons were infected with lentiviruses encoding scr-shRNA or myo6-shRNA at DIV4-5, followed by co-immunostaining at DIV14. The colocalized pixels of red (TOM20) and green (SV2) channels are highlighted in the white/black images. Data were quantified from the total number of neurons indicated within bars from three experiments. Data are expressed as mean ± SEM and analyzed by two-sided unpaired Student’s t-test. Scale bars: 20 μm (c) and 10 μm (g).
Extended Data Fig. 4 LatB Disrupts F-Actin without Impacting Density of Presynaptic Boutons and Axonal Mitochondria.
a, Disruption of F-actin with LatB. Cortical neurons were treated with DMSO or LatB (2 μM) for 3 hr at DIV14, followed by phalloidin labeling of F-actin and immunostaining of MAP2. b, STED images showing reduced F-actin in axons and at presynaptic terminals upon LatB treatment. Neurons at DIV14 were treated with DMSO or LatB (2 μM) for 3 hr, followed by SiR-actin labeling and immunostaining. c, d, Images (c) and analyses (d) showing unchanged mitochondrial integrity following LatB treatment. Neurons at DIV14 were treated with DMSO or LatB (2 μM) for 3 hr, or CCCP (10 μM) for 30 min, followed by labeling with CMTMRos (100 nM) for 20 min and immunostaining of TOM20. Mitochondrial integrity was assessed by integrated intensity ratio of CMTMRos vs TOM20 and normalized to DMSO. e–g, LatB treatment does not affect presynaptic density in live (e) and fixed neurons (f) and the density and size of axonal mitochondria (g). For live imaging, neurons were co-transfected with GFP-synapsin and DsRed-mito at DIV7-8, and dual-channel imaging was performed at DIV14. For fixed neuron imaging (f, g), neurons at DIV14 were treated with DMSO or LatB (2 μM) for 3 hr, followed by co-immunostaining. The average number of presynaptic boutons per 100 μm was quantified from the total number of neurons indicated in bars (d) or in parentheses (e–g) where n represents number of neurons and v stands for number of presynaptic terminals (f) or mitochondria (g) from three experiments. Data are presented as box plots (min, max, median, 25th and 75th percentiles) with dots as individual values (e) or bar graphs of mean ± SEM (d, f, g), and analyzed by two-sided unpaired Student’s t-test (e) or one-way ANOVA followed by Dunnett’s test (d, f, g). Scale bars: 10 μm (a,c) or 5 μm (b).
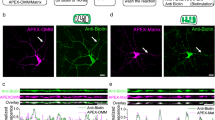
Extended Data Fig. 5 SNPH and F-actin Are Essential for Capturing Presynaptic Mitochondria.
a–c, Representative kymographs (a, b) and quantitative analyses (c) showing axonal mitochondria moving out of presynaptic terminals upon F-actin disruption in live neurons. Cortical neurons were co-transfected with GFP-synapsin and DsRed-Mito at DIV7-8, followed by dual-channel live imaging in distal axons at DIV14 (450 frames with 10-sec intervals for 75 min). After 90 frames (15 min) of live imaging, LatB was added to the chamber (10 μM) and the same axons were continuously time-lapse imaged for an additional 60 min. White arrows (a) point to mitochondria being released from presynaptic terminals; black arrows (b) point to presynaptic terminals capturing mitochondria under DMSO condition (b, left panels) or releasing mitochondria upon LatB treatment (b, right panels). The percentage of presynaptic terminals capturing mitochondria was quantified before and after LatB treatment. Note that disrupting F-actin reduces the percentage of presynaptic terminals capturing mitochondria. d, e, Electron micrographs (d) and quantitative analysis (e) showing reduced mitochondrial positioning at AZs upon F-actin disruption or snph deletion. WT or snph KO cortical neurons at DIV14 were treated with DMSO or LatB (2 μM) for 3 hr, followed by TEM. Yellow area shows AZs with SV clustering; green area shows mitochondria. Data were quantified from the total number of neurons (c) or AZs (e) per condition indicated in parentheses (c, e). Data are presented as box plots (min, max, median, 25th and 75th percentiles) with dots as individual values (c) or as bar graphs of mean percentage (e), and analyzed by two-sided Wilcoxon matched-pairs rank test (c). Scale bars: 10 μm (a, b) or 500 nm (d).
Extended Data Fig. 6 Deletion of SNPH or Knockdown of Myo6 Does Not Affect Axonal Cytoskeleton.
Representative STED super-resolution images showed that deleting snph (a) or knocking down myo6 (b) does not affect global structures of axonal cytoskeleton. WT or snph KO cortical neurons at DIV14 were immunostained by antibody against βIII-tubulin, followed by SiR-actin labeling of F-actin (a), or were infected with lentiviruses encoding scr-shRNA or myo6-shRNA at DIV5 and immunostained with βIII-tubulin, followed by SiR-actin labeling at DIV14 (b). Scale bars: 5 μm.
Extended Data Fig. 7 Modulating PAK-myo6 Axis Does Not Affect Density of Presynaptic Terminals or Mitochondrial Integrity.
Representative images (a, c) and quantitative analyses (b, d) showing no significant effect on the density of presynaptic terminals (a, b) and mitochondrial integrity (c,d) upon activation of PAK-myo6 axis by expressing PAK-CA or myo6-T405E. Cortical neurons at DIV7 were infected with lentiviruses encoding PAK3-CA or myo6-T405E, followed by co-immunostaining of synaptophysin (SYP) and βIII-tubulin (a, b) or by labeling mitochondria membrane potential with TMRE (50 nM for 20 min) along with total mitochondrial marker mitoTracker Green (20 nM for 20 min) at DIV14 (c, d). Mitochondrial integrity was assessed by the integrated intensity ratio of TMRE (red) vs mitoTracker (green) within individual mitochondria and normalized to the control group. Data were quantified from the total number of neurons indicated within bars, expressed as mean ± SEM, and analyzed by one-way ANOVA with post hoc testing by Dunnett’s multiple comparisons test. Scale bars: 10 μm.
Extended Data Fig. 8 Modulating the AMPK-PAK-myo6 Axis Has No Impact on Basal Synaptic Transmission.
Representative EPSC curves (a, c, e, g) and mean amplitudes (b, d, f, h) at 0.05 Hz showed no significant differences of basal synaptic transmission following modulation of the AMPK-PAK-myo6 pathway. Dual whole-cell patch-clamp recording was performed at DIV14-18 on paired cortical neurons treated with AICAR (1 mM) or CC (10 μM) for 2 hr (a, b); or presynaptic neurons expressing GFP, GFP-tagged myo6 or myo6-CBD (c,d); myo6-T405A or myo6-T405E (e, f); PAK3-KD or PAK3-CA (g, h). Data were quantified from the total pairs of neurons indicated in bars, represented as mean ± SEM, and analyzed by one-way ANOVA (b: F2, 72 = 1.764, P = 0.178; d: F2, 104 = 0.060, P = 0.941) with post hoc testing by Dunnett’s multiple comparisons test or two-sided unpaired Student’s t-test (f: P = 0.1162; h: P = 0.7052).
Extended Data Fig. 9 Disrupting SNPH-mediated Anchoring Abolishes the Role of PAK-myo6 in Maintaining Synaptic Transmission.
a, b, Normalized EPSC amplitudes plotted against stimulus number showing that activation of PAK-myo6 pathway facilitates short-term synaptic depression in WT (a), but not in snph KO neurons (b). Dual whole-cell patch-clamp recordings were performed on paired WT or snph KO cortical neurons infected with lentiviruses encoding GFP, GFP-tagged myo6, myo6-T405E. High-frequency (50 Hz, 200 msec) pulse trains were delivered to presynaptic neurons. Two-way ANOVA revealed a main effect of gene manipulation in WT neurons (F2, 61 = 5.319, P = 0.0074) (a), but no main effect in snph KO neurons (F2, 73 = 0.08927, P = 0.9147) (b). Data were quantified from total pairs of neurons indicated in parentheses and expressed as mean ± SEM. (c) Activation of PAK-myo6 signaling axis in snph KO neurons failed to accelerate synaptic recovery after high-frequency stimulation. snph KO cortical neurons were infected with lentiviruses encoding GFP, GFP-tagged myo6-WT, myo6-T405E, or PAK3-CA at DIV5, following by dual whole-cell patch clamp recording at DIV14-18. Recording configuration (total 132 sec duration) consists of a 2-sec 100 Hz train, a 10-sec recovery phase of four stimuli, and a 120-sec rest phase. The normalized EPSCs amplitude is plotted for the first of each HFS and for each of the recovery EPSCs for each condition. Two-way ANOVA revealed no main effect of gene manipulation in snph KO neurons (F3, 57 = 0.5787, P = 0.6314). Data were quantified from total pairs of neurons indicated in parentheses and expressed as mean ± SEM.
Extended Data Fig. 10 Presynaptic Mitochondria Contribute to the Recovery of Synaptic Efficacy During Prolonged Synaptic Activity.
a, Normalized EPSC amplitudes showing mitochondrial capacity of calcium clearance contributes to synaptic recovery after high-frequency stimulation (HFS). Cortical neurons were used for dual whole-cell patch clamp recording at DIV14-18. The mitochondrial calcium uniporter inhibitor RU360 (10 μM) was added to the intracellular recording solution to block mitochondrial calcium buffering capacity. Recording configuration (total 132 sec duration) consisted of a 2-sec 100 Hz train, a 10-sec recovery phase of four stimuli, and a 120-sec rest phase. The normalized EPSC amplitude was plotted for the first of each HFS and for each of the recovery EPSCs under each condition. Note that the application of RU360 induced a slower synaptic recovery after HFS. Two-way ANOVA revealed a main effect of RU360 treatment (F1, 41 = 10.64, P = 0.0022) and a significant interaction between RU360 treatment and stimuli (F14, 574 = 9.457, P < 0.001). b, Normalized EPSC amplitudes showing that myo6-SNPH-captured presynaptic mitochondria significantly contribute to synaptic recovery after HFS even their calcium buffering capacity is blocked. Cortical neurons were infected with lentiviruses encoding GFP, GFP-tagged myo6 and myo6-T405E, followed by dual whole-cell patch clamp recording at DIV14-18. 10 μM RU360 was applied to intracellular recording solution. The same recording configuration was used as described above. Note that enhanced presynaptic mitochondria anchoring through activating myo6-PAK pathway accelerates synaptic recovery after HFS even when calcium buffering capacity is blocked. Two-way ANOVA revealed a main effect of gene manipulation (F2, 60 = 3.506, P = 0.0363) and a significant interaction between gene manipulation and stimuli (F28, 840 = 2.074, P = 0.001). Data were quantified from the total pairs of neurons indicated in parentheses, and presented as mean ± SEM.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed Western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed Western blots and gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed Western blots and gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Unprocessed Western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Li, S., Xiong, GJ., Huang, N. et al. The cross-talk of energy sensing and mitochondrial anchoring sustains synaptic efficacy by maintaining presynaptic metabolism. Nat Metab 2, 1077–1095 (2020). https://doi.org/10.1038/s42255-020-00289-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-00289-0
This article is cited by
-
Insulin signalling regulates Pink1 mRNA localization via modulation of AMPK activity to support PINK1 function in neurons
Nature Metabolism (2024)
-
The AMPK-related kinase NUAK1 controls cortical axons branching by locally modulating mitochondrial metabolic functions
Nature Communications (2024)
-
Axonal energy metabolism, and the effects in aging and neurodegenerative diseases
Molecular Neurodegeneration (2023)
-
The multiple links between actin and mitochondria
Nature Reviews Molecular Cell Biology (2023)
-
mcPGK1-dependent mitochondrial import of PGK1 promotes metabolic reprogramming and self-renewal of liver TICs
Nature Communications (2023)