Abstract
Ketone bodies are essential alternative fuels that allow humans to survive periods of glucose scarcity induced by starvation and prolonged exercise. A widely used ketogenic diet (KD), which is extremely high in fat with very low carbohydrates, drives the host into using β-hydroxybutyrate for the production of ATP and lowers NLRP3-mediated inflammation. However, the extremely high fat composition of KD raises the question of how ketogenesis affects adipose tissue to control inflammation and energy homeostasis. Here, by using single-cell RNA sequencing of adipose-tissue-resident immune cells, we show that KD expands metabolically protective γδ T cells that restrain inflammation. Notably, long-term ad libitum KD feeding in mice causes obesity, impairs metabolic health and depletes the adipose-resident γδ T cells. In addition, mice lacking γδ T cells have impaired glucose homeostasis. Our results suggest that γδ T cells are mediators of protective immunometabolic responses that link fatty acid–driven fuel use to reduced adipose tissue inflammation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Code availability
Codes are publicly available in the relevant citations and custom script is available on request.
References
Moreno, B. et al. Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine 47, 793–805 (2014).
Moreno, B., Crujeiras, A. B., Bellido, D., Sajoux, I. & Casanueva, F. F. Obesity treatment by very low-calorie-ketogenic diet at two years: reduction in visceral fat and on the burden of disease. Endocrine 54, 681–690 (2016).
Shimazu, T. et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Goldberg, E. L. et al. Beta-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 18, 2077–2087 (2017).
Lu, Y. et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-kappaB signaling pathways. Neurosci. Lett. 683, 13–18 (2018).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Ageing and Health Fact Sheet 404 (World Health Organization, 2015).
Kanneganti, T. D. & Dixit, V. D. Immunological complications of obesity. Nat. Immunol. 13, 707–712 (2012).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Yang, H. et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J. Immunol. 185, 1836–1845 (2010).
Uysal, K. T., Wiesbrock, S. M., Marino, M. W. & Hotamisligil, G. S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389, 610–614 (1997).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 (2011).
Youm, Y. H. et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269 (2015).
Cahill, G. F. Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 26, 1–22 (2006).
Simeone, T. A., Simeone, K. A. & Rho, J. M. Ketone bodies as anti-seizure agents. Neurochem. Res. 42, 2011–2018 (2017).
Newman, J. C. et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 26, 547–557 e548 (2017).
Roberts, M. N. et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 26, 539–546 e535 (2017).
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017).
Youm, Y. H. et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18, 519–532 (2013).
Puchalska, P. et al. Hepatocyte-macrophage acetoacetate shuttle protects against tissue fibrosis. Cell Metab. 29, 383–398 (2018).
Kennedy, A. R. et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol. Metab. 292, E1724–E1739 (2007).
Srivastava, S., Baxa, U., Niu, G., Chen, X. & Veech, R. L. A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life 65, 58–66 (2013).
Kohlgruber, A. C. et al. Gammadelta T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol. 19, 464–474 (2018).
Badman, M. K. et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 5, 426–437 (2007).
Zhang, Y. et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1, e00065 (2012).
Paget, C. et al. CD3bright signals on gammadelta T cells identify IL-17A-producing Vgamma6Vdelta1+ T cells. Immunol. Cell Biol. 93, 198–212 (2015).
Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Ann. Rev. Pathol. 5, 99–118 (2010).
Wiley, C. D. et al. Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell 16, 1043–1050 (2017).
Costanzo, A. E. et al. Obesity impairs gammadelta T cell homeostasis and antiviral function in humans. PLoS ONE 10, e0120918 (2015).
Taylor, K. R., Mills, R. E., Costanzo, A. E. & Jameson, J. M. Gammadelta T cells are reduced and rendered unresponsive by hyperglycemia and chronic TNFalpha in mouse models of obesity and metabolic disease. PLoS ONE 5, e11422 (2010).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Edwards, C. et al. d-beta-hydroxybutyrate extends lifespan in C. elegans. Aging 6, 621–644 (2014).
Xie, Z. et al. Metabolic regulation of gene expression by histone lysine beta-hydroxybutyrylation. Mol. Cell 62, 194–206 (2016).
Garbow, J. R. et al. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G956–G967 (2011).
Ellenbroek, J. H. et al. Long-term ketogenic diet causes glucose intolerance and reduced beta- and alpha-cell mass but no weight loss in mice. Am. J. Physiol. Endocrinol. Metab. 306, E552–E558 (2014).
Rosenbaum, M. et al. Glucose and lipid homeostasis and inflammation in humans following an isocaloric ketogenic diet. Obesity 27, 971–981 (2019).
Sastre, J. et al. Aging of the liver: age-associated mitochondrial damage in intact hepatocytes. Hepatology 24, 1199–1205 (1996).
Wang, W. et al. A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab. 30, 174–189 e175 (2019).
Cheng, C. W. et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell 178, 1115–1131 e1115 (2019).
Wencker, M. et al. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat. Immunol. 15, 80–87 (2014).
Guo, X. J. et al. Lung gammadelta T cells mediate protective responses during neonatal influenza infection that are associated with type 2 immunity. Immunity 49, 531–544 e536 (2018).
Nielsen, M. M., Witherden, D. A. & Havran, W. L. Gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 17, 733–745 (2017).
Mehta, P., Nuotio-Antar, A. M. & Smith, C. W. Gammadelta T cells promote inflammation and insulin resistance during high fat diet-induced obesity in mice. J. Leukoc. Biol. 97, 121–134 (2015).
Zuniga, L. A. et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 185, 6947–6959 (2010).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Zhang, B. et al. Differential requirements of TCR signaling in homeostatic maintenance and function of dendritic epidermal T cells. J. Immunol. 195, 4282–4291 (2015).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Meth. 9, 676–682 (2012).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Meth. 9, 671–675 (2012).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Finak, G. et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278 (2015).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Mudge, J. M. & Harrow, J. Creating reference gene annotation for the mouse C57BL6/J genome assembly. Mamm. Genome 26, 366–378 (2015).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Karolchik, D. et al. The UCSC table browser data retrieval tool. Nucleic Acids Res. 32, D493–D496 (2004). (Database issue).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Sergushichev, A. An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. Preprint at bioRxiv https://doi.org/10.1101/060012 (2016).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Acknowledgements
We thank Y. Zhuang from the Duke University Medical School for providing the adipose tissue from Tcrd−/− reporter mice. We thank D. Gonzalez and A. Haberman for assistance with two-photon microscopy. E.L.G is funded by grant no. K99AG058801. The Dixit laboratory is supported in part by NIH grant nos. P01AG051459, AI105097, AR070811 and AG043608.
Author information
Authors and Affiliations
Contributions
E.L.G performed experiments and data analysis and prepared the manuscript. I.S. performed scRNA-seq analysis and assisted in manuscript preparation. J.L.A. performed parabiosis experiments and assisted in manuscript preparation. S.S. performed bulk RNA-seq analysis and assisted in manuscript preparation. M.N.A. conceived RNA-seq experiments, oversaw analyses and assisted in data interpretation. V.D.D. conceived the project, and helped with data interpretation and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Editor recognition Primary Handling Editor: Elena Bellafante.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
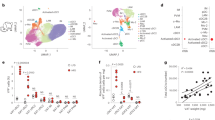
Extended Data Fig. 1 Workflow of single-cell RNAseq analysis.
Normalized gene expression from Efat tissue-resident CD45+ cells was used to identify most variable genes for principal component analysis. Data were visualized by tSNE plots. Unique markers were used to identify the cell type/lineage represented within each cluster.
Extended Data Fig. 2 Further identification of tissue-resident populations.
(a) tSNE plot of tissue-resident immune cells from chow- and KD-fed samples merged displaying expression of selected genes. (b) tSNE plot as in S2A showing average z-scores of genes in cell cycle pathway (Reactome database). (c) tSNE plot of proliferating cells only (cluster 10). Expression of selected markers is displayed. For (a-c) expression is based on pooled data from chow and KD samples (each containing n=3 pooled biological samples into 1 technical sample for each diet). (d) Violin plots of Il1b expression within all cells from each cluster. Expression is pooled from chow (n=4 pooled biological samples into 1 technical sample) and KD (n=3 pooled biological samples into 1 technical sample) and total number of cells in each cluster is indicated on the figure. Overlaid box plots indicate median and 25th-75th percentiles; whiskers extend no further than 1.5xIQR from either upper or lower hinge, as described in Materials and Methods.
Extended Data Fig. 3 Adipose immune compartment changes induced by 1 week of KD feeding.
Abundance of macrophages, eosinophils, and Tregs in Efat of chow (n=4) vs KD-fed (n=5) mice. Statistical differences were calculated by 2-way ANOVA with Sidak’s correction for multiple comparisons. Each symbol represents an individual mouse and all data are expressed as mean±SEM. Data are representative of at least 2 independent experiments. Exact p-values are shown whenever possible, ****p<0.0001.
Extended Data Fig. 4 γδ T cell expansion induced by KD is independent of NLRP3 and FGF21.
(a) Blood BHB levels, (b) Efat γδ T cells, and (c) spleen γδ T cells in Nlrp3−/- and Fgf21-/- mice after 1 week of KD feeding (n=6 biological independent mice per group). Statistical differences were calculated by unpaired 2-tailed t-tests within each genotype for each graph. All data are represented as mean±SEM and each symbol represents an individual mouse. Exact p-values are shown whenever possible, ****p<0.0001.
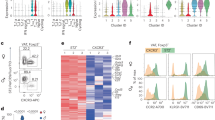
Extended Data Fig. 5 Workflow of bulk γδ T cell RNAseq analysis.
(a) Differentially-expressed genes were identified within all annotated transcribed murine gene loci. From this gene list we performed GSEA to distinguish pathways significantly altered by KD within epididymal adipose tissue γδ T cells. Differential expression gene list was further filtered to identify genes of interest. (b) GSEA enrichment score curve of SASP-related genes.
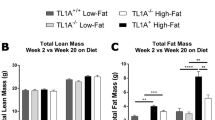
Extended Data Fig. 6 Long term KD feeding causes obesity in mice.
(a) Body weight, (b) lean mass, (c) fat mass, and (d) liver mass were measured in WT mice fed chow (n=8) or KD (n=9) for 4 months. For (a-d) Statistical differences were calculated by unpaired 2-tailed t-tests. Data are representative of 3 independent experiments. (e) Representative H&E-stained liver sections after long-term KD. Sections are representative of 2 independent experiments each with n=5 mice/group. (f) Western blot of liver AKT phosphorylation after insulin injection into fasted mice. Each lane represents an individual mouse. In KD group mice are ordered from greatest to smallest body weight (ranging from 59-37g). (g) Profile of visceral adipose hematopoietic compartment after 4 months chow (n=8) vs KD (n=8) feeding. Statistical differences were calculated by 2-way ANOVA with Sidak’s correction for multiple comparisons. All data are expressed as mean±SEM and each symbol represents an individual mouse. Exact p-values are shown whenever possible, ****p<0.0001.
Extended Data Fig. 7 Metabolic profiling of Tcrd-/- mice after long-term KD feeding.
(a) Lean mass and (b) body fat in WT (n=10) vs Tcrd-/- (n=11) mice after 4 months KD. Statistical differences were calculated by nonparametric Mann-Whitney 2-tailed test because the Tcrd-/- data are not normally distributed. Data are representative of 3 independent experiments. (c) Insulin tolerance test of WT (n=10) vs Tcrd-/- (n=7) mice after 3.5 months KD. Statistical differences were calculated by paired 2-way ANOVA. (d) Western blot of liver AKT phosphorylation after insulin injection into fasted mice. Each lane represents an individual mouse. (e) Total CD45+ cellularity in Efat from WT (n=11) vs Tcrd-/- (n=11) KD-fed mice. Statistical differences were determined by unpaired 2-tailed t-test. (f) CD45+ composition in epidydimal adipose tissue in WT (n=11) vs Tcrd-/- (n=11) mice after 4 months KD. Data are pooled from 2 independent experiments with a total of n=11 WT and n=11 Tcrd-/- mice analyzed. Statistical differences were calculated by 2-way ANOVA with Sidak’s correction for multiple comparisons. Each symbol represents an individual mouse and data are represented as mean±SEM. Exact p-values are shown whenever possible, ****p<0.0001.
Extended Data Fig. 8 Flow cytometry gating strategies.
Representative gating strategies are shown for each cell population analyzed throughout the experiments. (a) Gating strategy used to define all cell lineages analyzed in Fig. 4d, Fig. 6e, f, o, Extended Data Fig. 3, Extended Data Fig. 4b, c, Extended Data Fig. 6g, Extended Data Fig. 7e, f. (b) Gating strategy used to identify IL-17-producing γδ T cells and Tregs analyzed in Fig. 4h, Fig. 6p. (c) Gating strategy used to identify ILC2 analyzed in Extended Data Fig. 7c.
Extended Data Fig. 9 Comparison of bulk sorted γδ T cell RNAseq with scRNAseq dataset.
(a) tSNE plot of tissue-resident immune cells from chow- and KD-fed samples shown separately to demonstrate macrophage-specific genes differentially expressed in multiple lymphoid clusters as a result of macrophage frequency change (b) tSNE plot with overlaid color that represents expression of down-regulated genes identified from bulk RNAseq data if filtered only by padj. (c) tSNE plot with overlaid color that represents expression of down-regulated (left panel) and up-regulated (right panel) genes identified from bulk RNAseq data when filtered by padj and log2FC. For all plots (a-c) data are derived from a single technical sample generated by pooling n=3 independent samples prior to sequencing for each diet group.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2
Source data
Source Data Extended Data Fig. 6
Unprocessed western blots
Source Data Extended Data Fig. 7
Unprocessed western blots
Rights and permissions
About this article
Cite this article
Goldberg, E.L., Shchukina, I., Asher, J.L. et al. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat Metab 2, 50–61 (2020). https://doi.org/10.1038/s42255-019-0160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0160-6
This article is cited by
-
Macrophage and T cell networks in adipose tissue
Nature Reviews Endocrinology (2024)
-
Differential peripheral immune signatures elicited by vegan versus ketogenic diets in humans
Nature Medicine (2024)
-
Role of liver FGF21-KLB signaling in ketogenic diet-induced amelioration of hepatic steatosis
Nutrition & Diabetes (2024)
-
A ketogenic diet can mitigate SARS-CoV-2 induced systemic reprogramming and inflammation
Communications Biology (2023)
-
Interleukin-17 as a key player in neuroimmunometabolism
Nature Metabolism (2023)