Abstract
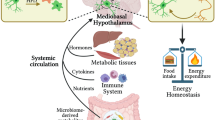
Tissue-resident myeloid cells initiate local inflammation in response to infectious or injurious stimuli. Sixteen years ago, macrophages in the adipose tissue (ATMs) were shown to undergo a form of activation in response to diet-induced obesity, thus leading to the conclusion that these macrophages sense a type of pro-inflammatory injury. ATMs are now known to be central to adipose tissue development, plasticity, maintenance and function. Indeed, their involvement in obesity may represent hijacking of these functions. More recently, microglia, ‘CNS macrophages’, have been shown to accumulate and undergo activation in response to dietary excess in the mediobasal hypothalamus (MBH), and early studies have implicated these cells as injury-responsive mediators of hypothalamic dysfunction. However, microglia are amazingly diverse cells now known to have moment-to-moment sensory functions and to communicate with neighbouring neurons to maintain and shape brain circuitry. Here, we build on this view, detailing our rapidly evolving understanding of microglial heterogeneity in the MBH and their roles as nutrient and environmental sensors. We propose that microglia, instead of simply responding to diet-induced damage, act as critical metabolic regulators that may coordinate a complex cellular network in the MBH. Understanding their roles in hypothalamic development and function should reveal unexpected mechanistic information relevant to important diseases such as obesity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Aguzzi, A., Barres, B. A. & Bennett, M. L. Microglia: scapegoat, saboteur, or something else? Science 339, 156–161 (2013).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012).
Valdearcos, M. et al. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 9, 2124–2138 (2014).
Kreutzer, C. et al. Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes 66, 2407–2415 (2017).
Valdearcos, M. et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 26, 185–197.e3 (2017).
Münzberg, H., Flier, J. S. & Bjørbaek, C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145, 4880–4889 (2004).
Scarpace, P. J. & Zhang, Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R493–R500 (2009).
Arkan, M. C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 (2005).
Han, M. S. et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339, 218–222 (2013).
Shimobayashi, M. et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Invest. 128, 1538–1550 (2018).
Xu, X. et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 18, 816–830 (2013).
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014).
Kratz, M. et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20, 614–625 (2014).
Robblee, M. M. et al. Saturated fatty acids engage an IRE1α-dependent pathway to activate the NLRP3 inflammasome in myeloid cells. Cell Rep. 14, 2611–2623 (2016).
Lancaster, G. I. et al. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab. 27, 1096–1110.e5 (2018).
Stevens, B. & Schafer, D. P. Roles of microglia in nervous system development, plasticity, and disease. Dev. Neurobiol. 78, 559–560 (2018).
Kuhn, S. A. et al. Microglia express GABA(B) receptors to modulate interleukin release. Mol. Cell. Neurosci. 25, 312–322 (2004).
Wang, W., Ji, P., Riopelle, R. J. & Dow, K. E. Functional expression of corticotropin-releasing hormone (CRH) receptor 1 in cultured rat microglia. J. Neurochem. 80, 287–294 (2002).
Santos-Carvalho, A., Aveleira, C. A., Elvas, F., Ambrósio, A. F. & Cavadas, C. Neuropeptide Y receptors Y1 and Y2 are present in neurons and glial cells in rat retinal cells in culture. Invest. Ophthalmol. Vis. Sci. 54, 429–443 (2013).
Mori, K. et al. Effects of norepinephrine on rat cultured microglial cells that express α1, α2, β1 and β2 adrenergic receptors. Neuropharmacology 43, 1026–1034 (2002).
Färber, K., Pannasch, U. & Kettenmann, H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol. Cell. Neurosci. 29, 128–138 (2005).
Pocock, J. M. & Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 30, 527–535 (2007).
Gao, Y. et al. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62, 17–25 (2014).
Yi, C.-X. et al. TNFα drives mitochondrial stress in POMC neurons in obesity. Nat. Commun. 8, 15143 (2017).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013).
Grabert, K. et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504–516 (2016).
Ransohoff, R. M. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 19, 987–991 (2016).
Prinz, M., Priller, J., Sisodia, S. S. & Ransohoff, R. M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 14, 1227–1235 (2011).
Gosselin, D. et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222 (2017).
Bennett, F. C. et al. A combination of ontogeny and CNS environment establishes microglial identity. Neuron 98, 1170–1183.e8 (2018).
Goldmann, T. et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 17, 797–805 (2016).
Jais, A. et al. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell 165, 882–895 (2016).
Lee, C. H. et al. Hypothalamic macrophage inducible nitric oxide synthase mediates obesity-associated hypothalamic inflammation. Cell Rep. 25, 934–946.e5 (2018).
André, C. et al. Inhibiting microglia expansion prevents diet-induced hypothalamic and peripheral inflammation. Diabetes 66, 908–919 (2017).
Noda, M. & Suzumura, A. Sweepers in the CNS: microglial migration and phagocytosis in the Alzheimer disease pathogenesis. Int. J. Alzheimers Dis. 2012, 891087 (2012).
Kim, C. et al. β1-integrin-dependent migration of microglia in response to neuron-released α-synuclein. Exp. Mol. Med. 46, e91 (2014).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395.e6 (2018).
Campbell, J. N. et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 20, 484–496 (2017).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017).
Haimon, Z. et al. Re-evaluating microglia expression profiles using RiboTag and cell isolation strategies. Nat. Immunol. 19, 636–644 (2018).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Gadani, S. P., Smirnov, I., Smith, A. T., Overall, C. C. & Kipnis, J. Characterization of meningeal type 2 innate lymphocytes and their response to CNS injury. J. Exp. Med. 214, 285–296 (2017).
Olofsson, L. E., Unger, E. K., Cheung, C. C. & Xu, A. W. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc. Natl Acad. Sci. USA 110, E697–E706 (2013).
Berkseth, K. E. et al. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology 155, 2858–2867 (2014).
Gao, Y. et al. Lipoprotein lipase maintains microglial innate immunity in obesity. Cell Rep. 20, 3034–3042 (2017).
Morselli, E. et al. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep. 9, 633–645 (2014).
Morselli, E. et al. A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. Int. J. Obes. (Lond.) 40, 206–209 (2016).
Dorfman, M. D. et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun. 8, 14556 (2017).
Hanamsagar, R. et al. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 65, 1504–1520 (2017).
Thion, M. S. et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500–516.e16 (2018).
Gao, Y. et al. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol. Metab. 6, 897–908 (2017).
Gao, Y. et al. Deficiency of leptin receptor in myeloid cells disrupts hypothalamic metabolic circuits and causes body weight increase. Mol. Metab. 7, 155–160 (2018).
Allison, M. B. et al. TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Mol. Metab. 4, 299–309 (2015).
Kim, J. G. et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 17, 908–910 (2014).
Wang, Y., Hsuchou, H., He, Y., Kastin, A. J. & Pan, W. Role of astrocytes in leptin signaling. J. Mol. Neurosci. 56, 829–839 (2015).
Cândido, F. G. et al. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 69, 125–143 (2018).
Sanz, Y., Rastmanesh, R. & Agostoni, C. Understanding the role of gut microbes and probiotics in obesity: how far are we? Pharmacol. Res. 69, 144–155 (2013).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Rosin, J. M., Vora, S. R. & Kurrasch, D. M. Depletion of embryonic microglia using the CSF1R inhibitor PLX5622 has adverse sex-specific effects on mice, including accelerated weight gain, hyperactivity and anxiolytic-like behaviour. Brain Behav. Immun. 73, 682–697 (2018).
Parlee, S. D. & MacDougald, O. A. Maternal nutrition and risk of obesity in offspring: the Trojan horse of developmental plasticity. Biochim. Biophys. Acta 1842, 495–506 (2014).
Bilbo, S. D. & Tsang, V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 24, 2104–2115 (2010).
Kang, S. S., Kurti, A., Fair, D. A. & Fryer, J. D. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflamm. 11, 156 (2014).
Bolton, J. L. & Bilbo, S. D. Developmental programming of brain and behavior by perinatal diet: focus on inflammatory mechanisms. Dialog-. Clin. Neurosci. 16, 307–320 (2014).
Wendeln, A.-C. et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338 (2018).
Schur, E. A. et al. Radiologic evidence that hypothalamic gliosis is associated withobesity and insulin resistance in humans. Obes. (Silver Spring) 23, 2142–2148 (2015).
Acknowledgements
The authors thank members of their respective laboratories for helpful discussions on the topic. This work was funded by the NIDDK (R01DK056731 to M.G.M., K01DK113064 to M.V. and R01DK098722 to S.K.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Valdearcos, M., Myers, M.G. & Koliwad, S.K. Hypothalamic microglia as potential regulators of metabolic physiology. Nat Metab 1, 314–320 (2019). https://doi.org/10.1038/s42255-019-0040-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0040-0
This article is cited by
-
Central activation of the fatty acid sensor GPR120 suppresses microglia reactivity and alleviates sickness- and anxiety-like behaviors
Journal of Neuroinflammation (2023)
-
Metabolic factors in the regulation of hypothalamic innate immune responses in obesity
Experimental & Molecular Medicine (2022)
-
Temporal profile of serum metabolites and inflammation following closed head injury in rats is associated with HPA axis hyperactivity
Metabolomics (2022)