Abstract
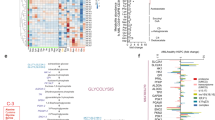
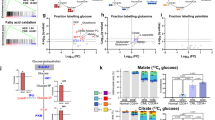
Amino acid metabolism is involved in diverse cellular functions, including cell survival and growth; however, it remains unclear how it regulates normal haematopoiesis versus leukaemogenesis. Here, we report that knockout of solute carrier family 1 member 5 (Slc1a5/ASCT2), a transporter of neutral amino acids, especially glutamine, results in mild-to-moderate defects in bone marrow and mature blood cell development under steady-state conditions. In contrast, constitutive or induced deletion of Slc1a5 decreases leukaemia initiation and maintenance driven by oncogene MLL-AF9 or phosphatase and tensin homologue (Pten) deficiency. Survival of leukaemic mice is prolonged following Slc1a5 deletion, and pharmacological inhibition of ASCT2 also decreases leukaemia development and progression in xenograft models of human acute myeloid leukaemia. Mechanistically, loss of ASCT2 generates a global effect on cellular metabolism, disrupts leucine (Leu) influx and mechanistic target of rapamycin (mTOR) signalling, and induces apoptosis in leukaemic cells. Given the substantial difference in reliance on ASCT2-mediated amino acid metabolism between normal and malignant blood cells, this in vivo study suggests ASCT2 as a promising therapeutic target for the treatment of leukaemia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The Reporting Summary for this article is available as a Supplementary Information file.
References
Hay, N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat. Rev. Cancer 16, 635–649 (2016).
Vander Heiden, M. G. & DeBerardinis, R. J. Understanding the intersections between metabolism and cancer biology. Cell 168, 657–669 (2017).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
DeNicola, G. M. & Cantley, L. C. Cancer’s fuel choice: new flavors for a picky eater. Mol. Cell 60, 514–523 (2015).
Carracedo, A., Cantley, L. C. & Pandolfi, P. P. Cancer metabolism: fatty acid oxidation in the limelight. Nat. Rev. Cancer 13, 227–232 (2013).
Altman, B. J., Stine, Z. E. & Dang, C. V. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 (2016).
Palm, W. & Thompson, C. B. Nutrient acquisition strategies of mammalian cells. Nature 546, 234–242 (2017).
Sassone-Corsi, P. Physiology. When metabolism and epigenetics converge. Science 339, 148–150 (2013).
Lu, C. & Thompson, C. B. Metabolic regulation of epigenetics. Cell Metab. 16, 9–17 (2012).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017).
Jewell, J. L., Russell, R. C. & Guan, K. L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 14, 133–139 (2013).
Kekuda, R. et al. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J. Biol. Chem. 271, 18657–18661 (1996).
Fuchs, B. C. & Bode, B. P. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin. Cancer Biol. 15, 254–266 (2005).
Hassanein, M. et al. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin. Cancer Res. 19, 560–570 (2013).
van Geldermalsen, M. et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 35, 3201–3208 (2016).
Wang, Q. et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 236, 278–289 (2015).
Willems, L. et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood 122, 3521–3532 (2013).
Schulte, M. L. et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat. Med. 24, 194–202 (2018).
Yu, W. M. et al. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell 12, 62–74 (2013).
Liu, X. et al. Maintenance of mouse hematopoietic stem cells ex vivo by reprogramming cellular metabolism. Blood 125, 1562–1565 (2015).
Oburoglu, L. et al. Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell 15, 169–184 (2014).
Kühn, R., Schwenk, F., Aguet, M. & Rajewsky, K. Inducible gene targeting in mice. Science 269, 1427–1429 (1995).
Velasco-Hernandez, T., Säwén, P., Bryder, D. & Cammenga, J. Potential pitfalls of the Mx1-Cre system: implications for experimental modeling of normal and malignant hematopoiesis. Stem Cell Rep. 7, 11–18 (2016).
Guo, W. et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature 453, 529–533 (2008).
Yilmaz, O. H. et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 (2006).
Zhang, J., Pavlova, N. N. & Thompson, C. B. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. 36, 1302–1315 (2017).
Jin, L., Alesi, G. N. & Kang, S. Glutaminolysis as a target for cancer therapy. Oncogene 35, 3619–3625 (2016).
Nicklin, P. et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136, 521–534 (2009).
Manifava, M. et al. Dynamics of mTORC1 activation in response to amino acids. eLife 5, e19960 (2016).
Esslinger, C. S., Cybulski, K. A. & Rhoderick, J. F. Ngamma-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg. Med Chem. 13, 1111–1118 (2005).
Nakaya, M. et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 40, 692–705 (2014).
Masle-Farquhar, E., Bröer, A., Yabas, M., Enders, A. & Bröer, S. ASCT2 (SLC1A5)-deficient mice have normal B-cell development, proliferation, and antibody production. Front. Immunol. 8, 549 (2017).
Locasale, J. W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013).
Maddocks, O. D. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546 (2013).
Durán, R. V. et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol. Cell 47, 349–358 (2012).
Cormerais, Y. et al. The glutamine transporter ASCT2 (SLC1A5) promotes tumor growth independently of the amino acid transporter LAT1 (SLC7A5). J. Biol. Chem. 293, 2877–2887 (2018).
Bröer, A., Rahimi, F. & Bröer, S. Deletion of amino acid transporter ASCT2 (SLC1A5) reveals an essential role for transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to sustain glutaminolysis in cancer cells. J. Biol. Chem. 291, 13194–13205 (2016).
Chiu, M. et al. GPNA inhibits the sodium-independent transport system L for neutral amino acids. Amino Acids 49, 1365–1372 (2017).
Dong, L. et al. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature 539, 304–308 (2016).
Xu, D. et al. Non-lineage/stage-restricted effects of a gain-of-function mutation in tyrosine phosphatase Ptpn11 (Shp2) on malignant transformation of hematopoietic cells. J. Exp. Med. 208, 1977–1988 (2011).
Acknowledgements
This work was supported by the National Institutes of Health grant nos. DK092722 and HL130995 (to C.K.Q).
Author information
Authors and Affiliations
Contributions
F.N. and W.M.Y. generated and characterized the haematopoietic cell development in Slc1a5 global and conditional knockout mice, set up the mouse leukaemia models and xenograft models of human AML and analysed leukaemia development/progression. F.N. also performed metabolic assays and rescue experiments. Z.L. performed the immunoblot analyses. L.J. performed the metabolite analyses. D.K.G. and S.L. provided patient specimens and discussed the work. M.R.R. and L.J. conducted TCGA and TARGET database mining and performed the correlation analyses. S.K. and H.E.B. provided critical advice on experimental design and interpretation of the data, and edited the manuscript. C.K.Q. designed the experiments and directed the entire project. F.N. and C.K.Q. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–13 and Supplementary Table 2
Supplementary Table 1
Metabolomic analysis data of ASCT2-deleted and ASCT2-undeleted Pten-deficient Lin– leukemic cells
Rights and permissions
About this article
Cite this article
Ni, F., Yu, WM., Li, Z. et al. Critical role of ASCT2-mediated amino acid metabolism in promoting leukaemia development and progression. Nat Metab 1, 390–403 (2019). https://doi.org/10.1038/s42255-019-0039-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0039-6
This article is cited by
-
Histidine re-sensitizes pediatric acute lymphoblastic leukemia to 6-mercaptopurine through tetrahydrofolate consumption and SIRT5-mediated desuccinylation
Cell Death & Disease (2024)
-
Nutrient transporters: connecting cancer metabolism to therapeutic opportunities
Oncogene (2023)
-
Targeting Glutamine Metabolism as an Attractive Therapeutic Strategy for Acute Myeloid Leukemia
Current Treatment Options in Oncology (2023)
-
MRI assessment of glutamine uptake correlates with the distribution of glutamine transporters and cancer stem cell markers
Scientific Reports (2022)
-
SLC38A4 functions as a tumour suppressor in hepatocellular carcinoma through modulating Wnt/β-catenin/MYC/HMGCS2 axis
British Journal of Cancer (2021)