Abstract
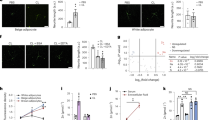
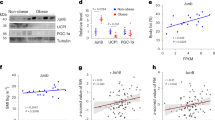
Thermogenesis is an important contributor to whole-body energy expenditure and metabolic homeostasis. Although circulating factors that promote energy expenditure are known, endocrine molecules that suppress energy expenditure have remained largely elusive. Here we found that Tsukushi (TSK) is a liver-enriched secreted factor that is highly inducible in response to increased energy expenditure. Hepatic Tsk expression and plasma TSK levels were elevated in obesity. In mice, TSK deficiency increased sympathetic innervation and norepinephrine release in adipose tissue, leading to enhanced adrenergic signalling and thermogenesis, attenuation of brown fat whitening, and protection from diet-induced obesity. Our data reveal TSK as part of a negative feedback mechanism that gates thermogenic energy expenditure and highlights TSK as a potential target for therapeutic intervention in metabolic disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The microarray dataset described in the paper has been deposited in the Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/) with accession number GSE114361. All other data are available from the corresponding author on reasonable request.
References
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Potthoff, M. J., Kliewer, S. A. & Mangelsdorf, D. J. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 26, 312–324 (2012).
Trujillo, M. E. & Scherer, P. E. Adipose tissue-derived factors: impact on health and disease. Endocr. Rev. 27, 762–778 (2006).
Waki, H. & Tontonoz, P. Endocrine functions of adipose tissue. Annu. Rev. Pathol. 2, 31–56 (2007).
Flier, J. S. & Maratos-Flier, E. Leptin’s physiologic role: does the emperor of energy balance have no clothes? Cell. Metab. 26, 24–26 (2017).
Friedman, J. 20 years of leptin: leptin at 20: an overview. J. Endocrinol. 223, T1–T8 (2014).
Staiger, H., Keuper, M., Berti, L., Hrabe de Angelis, M. & Haring, H. U. Fibroblast growth factor 21 - metabolic role in mice and men. Endocr. Rev. 38, 468–488 (2017).
Stefan, N. & Haring, H. U. The role of hepatokines in metabolism. Nat. Rev. Endocrinol. 9, 144–152 (2013).
Yanagi, S., Sato, T., Kangawa, K. & Nakazato, M. The homeostatic force of ghrelin. Cell. Metab. 27, 786–804 (2018).
Crewe, C., An, Y. A. & Scherer, P. E. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest. 127, 74–82 (2017).
Martinez-Santibanez, G. & Lumeng, C. N. Macrophages and the regulation of adipose tissue remodeling. Annu. Rev. Nutr. 34, 57–76 (2014).
Reilly, S. M. & Saltiel, A. R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13, 633–643 (2017).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Townsend, K. L. & Tseng, Y. H. Brown fat fuel utilization and thermogenesis. Trends Endocrinol. Metab. 25, 168–177 (2014).
Wu, J., Cohen, P. & Spiegelman, B. M. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 27, 234–250 (2013).
Ikeda, K. et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 23, 1454–1465 (2017).
Ikeda, K., Maretich, P. & Kajimura, S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Metab. 29, 191–200 (2018).
Kazak, L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015).
Chen, Z. et al. Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Mol. Metab. 6, 863–872 (2017).
Guo, L. et al. Hepatic neuregulin 4 signaling defines an endocrine checkpoint for steatosis-to-NASH progression. J. Clin. Invest. 127, 4449–4461 (2017).
Wang, G. X. et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 20, 1436–1443 (2014).
Lowell, B. B. et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366, 740–742 (1993).
Yoneshiro, T. et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123, 3404–3408 (2013).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205 (2011).
van der Lans, A. A. et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123, 3395–3403 (2013).
Cho, K. W., Zhou, Y., Sheng, L. & Rui, L. Lipocalin-13 regulates glucose metabolism by both insulin-dependent and insulin-independent mechanisms. Mol. Cell. Biol. 31, 450–457 (2011).
Meex, R. C. et al. Fetuin B is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell. Metab. 22, 1078–1089 (2015).
Ohba, K. et al. Desensitization and incomplete recovery of hepatic target genes after chronic thyroid hormone treatment and withdrawal in male adult mice. Endocrinology 157, 1660–1672 (2016).
Hossain, M. et al. The combinatorial guidance activities of draxin and Tsukushi are essential for forebrain commissure formation. Dev. Biol. 374, 58–70 (2013).
Ito, A. et al. Tsukushi is required for anterior commissure formation in mouse brain. Biochem. Biophys. Res. Commun. 402, 813–818 (2010).
Ohta, K. et al. Tsukushi functions as an organizer inducer by inhibition of BMP activity in cooperation with chordin. Dev. Cell. 7, 347–358 (2004).
de Jesus, L. A. et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J. Clin. Invest. 108, 1379–1385 (2001).
Zhao, X. Y. et al. Long noncoding RNA licensing of obesity-linked hepatic lipogenesis and NAFLD pathogenesis. Nat. Commun. 9, 2986 (2018).
Bartness, T. J., Liu, Y., Shrestha, Y. B. & Ryu, V. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocrinol. 35, 473–493 (2014).
Morrison, S. F. & Madden, C. J. Central nervous system regulation of brown adipose tissue. Compr. Physiol. 4, 1677–1713 (2014).
Zeng, W. et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 (2015).
Bachman, E. S. et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 (2002).
Bray, G. A. & York, D. A. The MONA LISA hypothesis in the time of leptin. Recent Prog. Horm. Res. 53, 95–117 (1998); discussion 117–118.
Cao, Y., Wang, H., Wang, Q., Han, X. & Zeng, W. Three-dimensional volume fluorescence-imaging of vascular plasticity in adipose tissues. Mol. Metab. 14, 71–81 (2018).
Chi, J. et al. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell. Metab. 27, 226–236 e223 (2018).
Jiang, H., Ding, X., Cao, Y., Wang, H. & Zeng, W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell. Metab. 26, 686–692 e683 (2017).
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Zhao, X. Y., Li, S., Wang, G. X., Yu, Q. & Lin, J. D. A long noncoding RNA transcriptional regulatory circuit drives thermogenic adipocyte differentiation. Mol. Cell 55, 372–382 (2014).
Li, S. et al. Genome-wide coactivation analysis of PGC-1alpha identifies BAF60a as a regulator of hepatic lipid metabolism. Cell. Metab. 8, 105–117 (2008).
Muller, H., Dai, G. & Soares, M. J. Placental lactogen-I (PL-I) target tissues identified with an alkaline phosphatase-PL-I fusion protein. J. Histochem. Cytochem. 46, 737–743 (1998).
Lin, J. & Linzer, D. I. Induction of megakaryocyte differentiation by a novel pregnancy-specific hormone. J. Biol. Chem. 274, 21485–21489 (1999).
Acknowledgements
This work was supported by NIH grants (nos. DK102456 and AG055379 to J.D.L.; no. DK114220 to L.R.), the Michigan Diabetes Research Center (grant no. DK020572), and the Michigan Nutrition and Obesity Center (grant no. DK089503).
Author information
Authors and Affiliations
Contributions
J.D.L. and Q.W. conceived the project and designed the research. Q.W., V.P.S., H.S., Y.X., Q.Z., X.X., L.G., H. S., S.L., L.R., and L.J. performed the experiments and analysed the data. K.O. provided the Tsk knockout mouse strain. J.D.L. and Q.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–8 and Supplementary Table 3
Supplementary Table 1
Microarray expression values for mouse secretome genes
Supplementary Table 2
List of liver-enriched secretome genes
Rights and permissions
About this article
Cite this article
Wang, Q., Sharma, V.P., Shen, H. et al. The hepatokine Tsukushi gates energy expenditure via brown fat sympathetic innervation. Nat Metab 1, 251–260 (2019). https://doi.org/10.1038/s42255-018-0020-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-018-0020-9
This article is cited by
-
Reduced hepatic bradykinin degradation accounts for cold-induced BAT thermogenesis and WAT browning in male mice
Nature Communications (2023)
-
Association of serum Tsukushi level with metabolic syndrome and its components
Endocrine (2023)
-
The muscle-enriched myokine Musclin impairs beige fat thermogenesis and systemic energy homeostasis via Tfr1/PKA signaling in male mice
Nature Communications (2023)
-
Hepatocellular carcinoma induced by hepatocyte Pten deletion reduces BAT UCP-1 and thermogenic capacity in mice, despite increasing serum FGF-21 and iWAT browning
Journal of Physiology and Biochemistry (2023)
-
Blocking ActRIIB and restoring appetite reverses cachexia and improves survival in mice with lung cancer
Nature Communications (2022)