Abstract
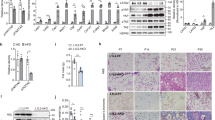
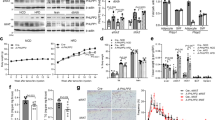
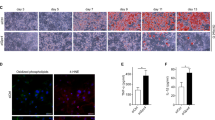
Decreased adipose tissue oxygen tension and increased expression of the transcription factor hypoxia-inducible factor–1α (HIF-1α) can trigger adipose tissue inflammation and dysfunction in obesity. Our current understanding of obesity-associated decreased adipose tissue oxygen tension is mainly focused on changes in oxygen supply and angiogenesis. Here, we demonstrate that increased adipocyte oxygen demand, mediated by activity of the mitochondrial protein adenine nucleotide translocase 2 (ANT2), is the dominant cause of adipocyte hypoxia. Deletion of adipocyte Ant2 (also known as Scl25a5) improves obesity-induced intracellular adipocyte hypoxia by decreasing obesity-induced adipocyte oxygen demand, without effects on mitochondrial number or mass, or oligomycin-sensitive respiration. This effect of adipocyte ANT2 knockout led to decreased adipose tissue HIF-1α expression and inflammation with improved glucose tolerance and insulin resistance in both preventative and therapeutic settings. Our results suggest that ANT2 may be a target for the development of insulin-sensitizing drugs and that ANT2 inhibition might have clinical utility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data that support the findings of this study are included in the paper or its supplementary information.
References
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Kusminski, C. M., Bickel, P. E. & Scherer, P. E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug. Discov. 15, 639–660 (2016).
Lee, Y. S., Wollam, J. & Olefsky, J. M. An integrated view of immunometabolism. Cell 172, 22–40 (2018).
Friedman, J. The long road to leptin. J. Clin. Invest. 126, 4727–4734 (2016).
Yore, M. M. et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332 (2014).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Cao, H. et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134, 933–944 (2008).
Ferrante, A. W. Jr. The immune cells in adipose tissue. Diabetes Obes. Metab. 15 Suppl. 3, 34–38 (2013).
Ying, W. et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 171, 372–384 e312 (2017).
Li, P. et al. Hematopoietic-derived galectin-3 causes cellular and systemic insulin resistance. Cell 167, 973–984 e912 (2016).
Schwartz, D. R. & Lazar, M. A. Human resistin: found in translation from mouse to man. Trends Endocrinol. Metab. 22, 259–265 (2011).
Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 (2006).
Solinas, G. et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 6, 386–397 (2007).
Saberi, M. et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 10, 419–429 (2009).
Patsouris, D. et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8, 301–309 (2008).
Lee, Y. S. et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60, 2474–2483 (2011).
Lee, Y. S. et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014).
Halberg, N. et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 29, 4467–4483 (2009).
Hosogai, N. et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56, 901–911 (2007).
Palazon, A., Goldrath, A. W., Nizet, V. & Johnson, R. S. HIF transcription factors, inflammation, and immunity. Immunity 41, 518–528 (2014).
Jiang, C. et al. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 60, 2484–2495 (2011).
Lee, K. Y., Gesta, S., Boucher, J., Wang, X. L. & Kahn, C. R. The differential role of Hif1β/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab. 14, 491–503 (2011).
Gealekman, O. et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 123, 186–194 (2011).
Cao, Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 18, 478–489 (2013).
Sun, K., Halberg, N., Khan, M., Magalang, U. J. & Scherer, P. E. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell. Biol. 33, 904–917 (2013).
Pasarica, M. et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 (2009).
Keith, B., Johnson, R. S. & Simon, M. C. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 12, 9–22 (2011).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Amano, S. U. et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 19, 162–171 (2014).
Haase, J. et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia 57, 562–571 (2014).
Regula, K. M., Ens, K. & Kirshenbaum, L. A. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ. Res. 91, 226–231 (2002).
Kubli, D. A., Ycaza, J. E. & Gustafsson, A. B. Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem. J. 405, 407–415 (2007).
Vande Velde, C. et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol. Cell. Biol. 20, 5454–5468 (2000).
Chavez, J. A. & Summers, S. A. A ceramide-centric view of insulin resistance. Cell Metab. 15, 585–594 (2012).
Holland, W. L. et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 (2011).
Andreyev, A. et al. The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur. J. Biochem. 182, 585–592 (1989).
Fabbrini, E. et al. Metabolically normal obese people are protected from adverse effects following weight gain. J. Clin. Invest. 125, 787–795 (2015).
McLaughlin, T. et al. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obes. (Silver Spring) 22, 673–680 (2014).
Kolehmainen, M., Vidal, H., Alhava, E. & Uusitupa, M. I. Sterol regulatory element binding protein 1c (SREBP-1c) expression in human obesity. Obes. Res. 9, 706–712 (2001).
Pasarica, M. et al. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J. Clin. Endocrinol. Metab. 95, 4052–4055 (2010).
Li, P. et al. Adipocyte NCoR knockout decreases PPARγ phosphorylation and enhances PPARγ activity and insulin sensitivity. Cell 147, 815–826 (2011).
Kusminski, C. M. et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 18, 1539–1549 (2012).
Kim, J. Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007).
Liu, Y. & Chen, X. J. Adenine nucleotide translocase, mitochondrial stress, and degenerative cell death. Oxid. Med. Cell. Longev. 2013, 146860 (2013).
Shabalina, I. G., Kramarova, T. V., Nedergaard, J. & Cannon, B. Carboxyatractyloside effects on brown-fat mitochondria imply that the adenine nucleotide translocator isoforms ANT1 and ANT2 may be responsible for basal and fatty-acid-induced uncoupling respectively. Biochem. J. 399, 405–414 (2006).
Cho, J. et al. Mitochondrial ATP transporter depletion protects mice against liver steatosis and insulin resistance. Nat. Commun. 8, 14477 (2017).
Kokoszka, J. E. et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427, 461–465 (2004).
Seo, J. B. et al. Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor gamma expression. Mol. Cell. Biol. 24, 3430–3444 (2004).
Farrall, A. L. & Whitelaw, M. L. The HIF1α-inducible pro-cell death gene BNIP3 is a novel target of SIM2s repression through cross-talk on the hypoxia response element. Oncogene 28, 3671–3680 (2009).
Quispe-Tintaya, W. et al. Fast mitochondrial DNA isolation from mammalian cells for next-generation sequencing. Biotechniques 55, 133–136 (2013).
Don, A.S. & Rosen, H. A fluorescent plate reader assay for ceramide kinase. Anal. Biochem. 375, 265–271 (2008).
Acknowledgements
We thank D. Wallace (University of Pennsylvania) for providing Ant2 floxed mice. This study was supported by the US National Institute of Diabetes and Digestive and Kidney Diseases (DK063491 and DK101395), a University of California San Diego/University of California Los Angeles Diabetes Research Center P&F grant, the Basic Science Research Program and the Bio-Synergy Research Project through National Research Foundation of Korea (NRF-2017R1C1B2011125 and NRF-2017M3A9C4065956), the POSCO TJ Park Foundation, and a grant from the Merck, Inc., Janssen Pharmceuticals, Inc., and Pershing Square Foundation. M.R. was supported by a postdoctoral fellowship from the American Heart Association (16POST29990015).
Author information
Authors and Affiliations
Contributions
Y.S.L. and J.B.S. designed and performed the majority of the experiments. M.R. performed glucose clamp experiments. P.C. measured adipose tissue interstitial oxygen tension and hemodynamics. A.N.M. and A.Y.A supported measuring mitochondrial activity and oxygen consumption. J.Y.H. performed flow cytometry analysis of adipose tissue immune cells. S.C.B., G.I.S. and S.K. performed clinical studies in MNL, MAO and MNO subjects and measured human adipose tissue oxygen tension. Y.S.L. and J.M.O. conceived and supervised the project. J.B.S., Y.S.L. and J.M.O. wrote the manuscript and all authors commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1 and 2
Rights and permissions
About this article
Cite this article
Seo, J.B., Riopel, M., Cabrales, P. et al. Knockdown of ANT2 reduces adipocyte hypoxia and improves insulin resistance in obesity. Nat Metab 1, 86–97 (2019). https://doi.org/10.1038/s42255-018-0003-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-018-0003-x
Keywords
This article is cited by
-
PDGFRβ + cell HIF2α is dispensable for white adipose tissue metabolic remodeling and hepatic lipid accumulation in obese mice
Lipids in Health and Disease (2024)
-
Unraveling the complex roles of macrophages in obese adipose tissue: an overview
Frontiers of Medicine (2024)
-
The evolving functions of the vasculature in regulating adipose tissue biology in health and obesity
Nature Reviews Endocrinology (2023)
-
Chromogranin A-derived peptides pancreastatin and catestatin: emerging therapeutic target for diabetes
Amino Acids (2023)
-
Adipocyte-derived extracellular vesicles: bridging the communications between obesity and tumor microenvironment
Discover Oncology (2023)