Abstract
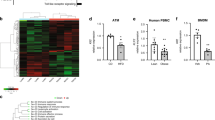
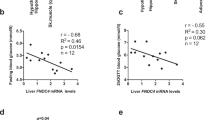
Enteroendocrine cells relay energy-derived signals to immune cells to signal states of nutrient abundance and control immunometabolism. Emerging data suggest that the gut-derived nutrient-induced incretin glucose-dependent insulinotropic polypeptide (GIP) operates at the interface of metabolism and inflammation. Here we show that high-fat diet (HFD)-fed mice with immune cell-targeted GIP receptor (GIPR) deficiency exhibit greater weight gain, insulin resistance, hepatic steatosis and significant myelopoiesis concomitantly with impaired energy expenditure and inguinal white adipose tissue (WAT) beiging. Expression of the S100 calcium-binding protein S100A8 was increased in the WAT of mice with immune cell-targeted GIPR deficiency and co-deletion of GIPR and the heterodimer S100A8/A9 in immune cells ameliorated the aggravated metabolic and inflammatory phenotype following a HFD. Specific GIPR deletion in myeloid cells identified this lineage as the target of GIP effects. Furthermore, GIP directly downregulated S100A8 expression in adipose tissue macrophages. Collectively, our results identify a myeloid–GIPR–S100A8/A9 signalling axis coupling nutrient signals to the control of inflammation and adaptive thermogenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The microarray datasets generated during the current study are deposited at the National Center for Biotechnology Information Gene Expression Omnibus public database under accession code GSE109371.
Change history
01 March 2019
In the version of the article originally published, Fig. 3a was missing a y-axis line and key labels, and Fig. 3c,d had illegible text labels overlapping the graphs. The errors have been corrected in the HTML and PDF versions of the article.
References
Gribble, F. M. & Reimann, F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 78, 277–299 (2016).
Monteiro, M. P. & Batterham, R. L. The importance of the gastrointestinal tract in controlling food intake and regulating energy balance. Gastroenterology 152, 1707–1717.e2 (2017).
Genton, L. & Kudsk, K. A. Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am. J. Surg. 186, 253–258 (2003).
Brestoff, J. R. & Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 161, 146–160 (2015).
Miyawaki, K. et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med. 8, 738–742 (2002).
Kim, S. J., Nian, C. & McIntosh, C. H. Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. J. Biol. Chem. 282, 8557–8567 (2007).
Gogebakan, O. et al. Glucose-dependent insulinotropic polypeptide reduces fat-specific expression and activity of 11β-hydroxysteroid dehydrogenase type 1 and inhibits release of free fatty acids. Diabetes 61, 292–300 (2012).
Kim, S. J. et al. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS ONE 7, e40156 (2012).
Varol, C. et al. Long-acting glucose-dependent insulinotropic polypeptide ameliorates obesity-induced adipose tissue inflammation. J. Immunol. 193, 4002–4009 (2014).
Baggio, L. L. & Drucker, D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157 (2007).
Campbell, J. E. & Drucker, D. J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell. Metab. 17, 819–837 (2013).
Pivovarova, O. et al. Regulation of nutrition-associated receptors in blood monocytes of normal weight and obese humans. Peptides 65, 12–19 (2015).
Suzuki, Y. et al. Anti-inflammatory role of glucose-dependent insulinotropic polypeptide in periodontitis. J. Diabetes Investig. 7, 497–505 (2016).
Nagashima, M. et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 54, 2649–2659 (2011).
Nogi, Y. et al. Glucose-dependent insulinotropic polypeptide prevents the progression of macrophage-driven atherosclerosis in diabetic apolipoprotein E-null mice. PLoS ONE 7, e35683 (2012).
Nagareddy, P. R. et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell. Metab. 19, 821–835 (2014).
Nagareddy, P. R. et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell. Metab. 17, 695–708 (2013).
Sekimoto, R. et al. Visualized macrophage dynamics and significance of S100A8 in obese fat. Proc. Natl. Acad. Sci. USA 112, E2058–E2066 (2015).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Ehrchen, J. M., Sunderkotter, C., Foell, D., Vogl, T. & Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 86, 557–566 (2009).
Manitz, M. P. et al. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol. Cell. Biol. 23, 1034–1043 (2003).
Reilly, S. M. & Saltiel, A. R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13, 633–643 (2017).
Pruenster, M., Vogl, T., Roth, J. & Sperandio, M. S100A8/A9: from basic science to clinical application. Pharmacol. Ther. 167, 120–131 (2016).
Campbell, J. E. et al. TCF1 links GIPR signaling to the control of beta cell function and survival. Nat. Med. 22, 84–90 (2016).
Ussher, J. R. et al. Inactivation of the glucose-dependent insulinotropic polypeptide receptor improves outcomes following experimental myocardial infarction. Cell Metab. 27, 450–460 (2018).
Foell, D., Wittkowski, H., Vogl, T. & Roth, J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol. 81, 28–37 (2007).
Kim, S. J. et al. A protein profile of visceral adipose tissues linked to early pathogenesis of type 2 diabetes mellitus. Mol. Cell. Proteomics 13, 811–822 (2014)..
Mortensen, O. H. et al. Calprotectin—a novel marker of obesity. PLoS ONE 4, e7419 (2009).
Catalan, V. et al. Increased levels of calprotectin in obesity are related to macrophage content: impact on inflammation and effect of weight loss. Mol. Med. 17, 1157–1167 (2011).
Finan, B. et al. Reappraisal of GIP pharmacology for metabolic diseases. Trends Mol. Med. 22, 359–376 (2016).
Frias, J. P. et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell. Metab. 26, 343–352.E2 (2017).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Wang, J., Vasaikar, S., Shi, Z., Greer, M. & Zhang, B. WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 45, 130–137 (2017).
Irizarry, R.A., et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Acknowledgements
The authors acknowledge support from the Israel Science Foundation for C.V. and S.F. (grant nos 35/12 and 1146/16) and the Canada Research Chairs Program and Canadian Institutes of Health Research Foundation grant 154321, both to D.J.D. Y.K. is the incumbent of the Sarah and Rolando Uziel Research Associate Chair. We thank A. H. Futerman and Y. Pewzner-Jung for their assistance with metabolic cages experiments. Finally, we thank N. Strauss (Sourasky Medical Center) for radiation services.
Author information
Authors and Affiliations
Contributions
F.D.M., I.Z., C.V. and S.F. conceived the study, designed experiments and wrote the manuscript. F.D.M., I.Z and C.V. performed the experiments and analysed the data. M.P-C. performed the bioinformatic analyses. T.V. analysed data, provided reagents and reviewed the manuscript. K.C., A.E. and S.W. performed many of the RT–PCR and immunoblot analyses in the presented experiments. Y.K. greatly assisted in the performance of metabolic cages experiments and analyses. D.J.D. provided key scientific consultation, essential reagents and mouse tools and carefully reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1 and 2
Rights and permissions
About this article
Cite this article
Mantelmacher, F.D., Zvibel, I., Cohen, K. et al. GIP regulates inflammation and body weight by restraining myeloid-cell-derived S100A8/A9. Nat Metab 1, 58–69 (2019). https://doi.org/10.1038/s42255-018-0001-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-018-0001-z
Keywords
This article is cited by
-
Senescent immune cells accumulation promotes brown adipose tissue dysfunction during aging
Nature Communications (2023)
-
Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1
Nature Reviews Endocrinology (2023)
-
Reversal of aging-associated increase in myelopoiesis and expression of alarmins by angiotensin-(1–7)
Scientific Reports (2023)
-
Pharmacological modulation of adaptive thermogenesis: new clues for obesity management?
Journal of Endocrinological Investigation (2023)
-
The expanding incretin universe: from basic biology to clinical translation
Diabetologia (2023)