Abstract
The notion of strong electronic correlations arose in the context of d-metal oxides such as NiO but can be exemplified on systems as simple as the H2 molecule. Here we shed light on correlation effects on B62− clusters as found in MB6 hexaborides and show that the B 2p valence electrons are fairly correlated. B6-octahedron excitation energies computed for CaB6 and YbB6 agree with peak positions found by resonant inelastic x-ray scattering, providing a compelling picture for the latter. Our findings characterize these materials as very peculiar p-electron correlated systems and call for more involved many-body investigations within the whole hexaboride family, both alkaline- and rare-earth compounds, not only for N- but also (N ± 1)-states defining e. g. band gaps.
Similar content being viewed by others
Introduction
The physical properties of MB6 hexaborides are remarkably diverse and have received persistent interest1,2, as a thorough understanding of this peculiar family of materials could not be achieved yet. Some of its members possess properties relevant for applications, generally determined by specificities of their electronic structures: CaB6 is a semiconductor3, YB6 turns superconducting for temperatures lower than 8 K4, LaB6 is widely used as thermionic electron emitter5, EuB6 displays colossal magneto-resistance6, the heavy-fermion system Ce1-xLaxB6 has been intensively studied in the context of multipolar phases and magnetically hidden orders7,8, while SmB6 is believed to host correlated topological states9. However, even for the seemingly simplest member of the family, CaB6, there are important electronic-structure features that are not at all clear, for example, the size and nature of its band gap: density-functional computations yield a vanishing band gap10,11,12,13,14 while experiment indicates a gap of ~1 eV3. The band structures of SmB6 and YbB6 are also a topic of active debate, especially in relation to their surface states9,15,16,17, for SmB6, even predictions made for the symmetry of the Sm3+-ion ground-state term are recently contradicted by experiment18,19.
In this context, we argue that one aspect constantly neglected in the study of these compounds is correlations within the B 2p electronic subsystem. To address such physics, we here employ ab initio multi-configurational wavefunction theory. For CaB6 and YbB6, representative divalent hexaborides, the ground-state wavefunctions turn out strongly multi-configurational, with large admixture of higher-lying configurations. Trying to understand the nature of B 2p N-particle states, a rich N-particle excitation spectrum is evidenced in the ab initio calculations. These computed p-p excitation energies compare well with peak positions in B K-edge resonant inelastic x-ray spectra (RIXS) reported by Denlinger et al.3,20. Analysis of the many-body wavefunctions in terms of site-centered valence orbitals suggests that the ab initio data can be mapped onto a six-site, half-filled effective Hubbard model. Unfolded to the extended lattice, this might provide a convenient frame for further insights into the electronic properties of the B 2p subsystem. The computationally more expensive option is using quantum chemical methods to determine the nature of (N ± 1) quasi-particle states21,22,23 defining e. g. band gaps.
Results and discussion
Correlated electronic structure of CaB6
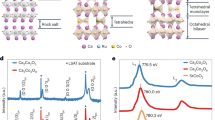
The crystal structure of cubic MB6 hexaborides is CsCl-like, with Pm-3m symmetry1,2. A given B6 octahedron is surrounded by eight M nearest-neighbor sites defining a cube (see Fig. 1). M can be an alkaline-earth atom, Y, or a rare-earth ion (e.g., La, Ce, Sm, Yb). Interestingly, even for CaB6, a conventional closed-shell p-electron compound at first sight, standard band-structure calculations and experiment provide conflicting results on the fundamental gap: computations within the local density approximation (LDA) of density functional theory, for instance, indicate a metallic ground state10,14 while a gap of ~1 eV is found experimentally3. One obvious question is then what kind of correlations are missed in local density approximation (LDA) based density functional theory in this class of materials, but the answer is not evident. From post-LDA GW calculations, both finite24 and vanishing13,14 band gaps were reported. These contradictory sets of data suggest that the results are sensitive to specific details in the numerical implementation. Moreover, the GW scheme is not designed for dealing with short-range correlations; the latter turn out to be massive, as discussed below.
For an isolated B atom, the Aufbau principle implies an [1s2]2s22p1 valence configuration; on a B62− octahedral unit, there are 6·3 + 2 = 20 electrons associated with the B 2s and 2p shells. But those 20 electrons can now be regarded as occupying symmetry-adapted orbitals distributed over several B sites of a given octahedron. In cubic geometry (i. e., regular octahedron), there are a1g, eg, and t1u symmetry-adapted functions associated with the six s atomic orbitals and a1g, eg, t1u, and t2g linear combinations related to the set of 18 p atomic orbitals.
To gain insight into the B 2s/2p electronic structure, we here rely on ab initio quantum chemical methods25 as implemented in the Orca program package v5.026. The material model is a point-charge embedded B62− cluster, for both CaB6 and YbB6 (see Fig. 1). Details on the technicalities of this embedding process are given in the Methods section. The quantum chemical investigation was initiated as a preliminary Hartree-Fock (HF) calculation that provides starting orbitals for a much more sophisticated post-HF treatment: complete-active-space self-consistent-field (CASSCF) computations and subsequent multi-reference configuration interaction (MRCI) with single and double excitations25. To make the highly demanding MRCI calculations feasible, we enabled the use of symmetry, according to the D2h subgroup; B 1s orbitals were not correlated.
Starting from the closed-shell HF solution, basic information on the nature of the low-lying excited states and on the nature of most important correlation effects was gathered from a series of CASSCF calculations in which different numbers of orbitals were considered as active. In quantum chemical terminology, “active” is the set of orbitals within which all possible determinants are generated on the basis of a given number of electrons. These are chemically or physically relevant orbitals, to e. g. bonding or spectroscopy, respectively; less relevant orbitals are assigned either double (core/semi-core levels) or zero (virtual levels) occupancy. By analyzing different active orbital spaces, the six molecular orbitals shown in Fig. 2(a) were found to be strongly correlated; in Oh point group symmetry, relevant to a B62− unit in cubic hexaborides, these orbitals transform according to the A1g, Eg, and T1u irreducible representations. We found that for the aspects discussed here a six-orbital active space (a1g + eg + t1u) accommodating six electrons (for lower-energy orbitals on the B62− octahedron, double occupancy is imposed in CASSCF) already provides a balanced description; we refer to this active space as CAS(6,6).
Using this active space, a total of one septet, five quintets, 15 triplets, and eight singlet states were considered in a state-averaged25 CASSCF calculation. Results computed this way for CaB6 are listed in Table 1. Electronic configurations are here expressed in terms of symmetry-adapted molecular-like orbitals (a1g, eg, t1u); this is common practice when discussing the electronic structure of octahedral atomic units and also the usual way output numerical data are printed by quantum chemical programs. A first observation is the pronounced multi-configurational structure of the computed wavefunctions. The leading ground-state configuration, for example, has a weight of only 61% in the many-body ground-state wavefunction (1A1g state) and a similar situation is observed for many of the excited states; the remaining weight has to do in each case with other electron configurations. The multi-configurational character of most of the states in Table 1 is also reflected in the natural-orbital occupation numbers, obtained through diagonalization of the density matrix25:for the 1A1g ground state, for instance, the a1g, eg, and t1u occupation numbers are 1.85, 1.70 (x2), and 0.25 (x3), respectively; at the HF level the occupation of the anti-bonding (see Fig. 2(a)) t1u levels is 0 since the HF ground-state wavefunction is 100% a1g2 eg4. Also illustrative for the effect of electronic correlations: the (restricted open-shell) HF 1A1g-5T2g splitting, for instance, is less than 1 eV, much smaller than the CASSCF/MRCI values provided in Table 1.
An instructive exercise is re-expressing the CAS(6,6) ground-state wavefunction in terms of site-centered orbitals. To achieve this, the symmetry-adapted a1g, eg, and t1u composites were transformed to a set of six symmetry-equivalent functions using the orbital “localization” module in Orca26. Two of these symmetry-equivalent site-centered orbitals are displayed in Fig. 2(b). Their asymmetric shape clearly indicates p-s mixing: dominant p but also s character, with substantial weight at the opposite B site. In this basis, the ground-state wavefunction amounts to 54% determinants with single orbital occupation, i.e., \(|\uparrow \downarrow \uparrow \downarrow \uparrow \downarrow \rangle\), 36% \(|\uparrow \downarrow \uparrow \downarrow 02\rangle\) + …, 7% \(|\uparrow \downarrow 0202\rangle\) + …, and 3% \(|020202\rangle\) + … configurations, where \(\uparrow\), \(\downarrow\), 0, and 2 stand for spin-up, spin-down electron, zero and double orbital occupation, respectively. As such, the wavefunction can in principle be mapped onto an effective six-site Hubbard-type model (t-U or t-U-V). Estimating hopping (t) and Coulomb repulsion (U, V) integrals27,28,29 defining such effective models is beyond the scope of this work. But the quantum chemical wavefunctions and excitation energies reported here provide useful ab initio benchmarks for tuning the relevant parameters. Remarkably, p-electron Hubbard-U values as large as 9 eV were estimated for e. g. carbon and phosphorus allotropes30,31.
Good insight into correlation effects on the B62− unit is attained by inspecting the wavefunction describing two opposite B sites (the hopping integral is larger for opposite-corner sp hybrids). To this end the following numerical test can be made: allow all possible occupations for two sp hybrids on opposite corners of the octahedron (c.f. those pictured in Fig. 2(b)) but restrict the occupation of each of the other four sp hybrids to 1 (without re-optimizing the orbitals). The weight of configurations with 0, 2 and 2, 0 orbital occupation for the opposite-corner orbitals is only 19%, indicative of sizable correlations. For comparison, in the H2 molecule at equilibrium H-H interatomic distance the weight of those ionic configurations in the HF wavefunction (uncorrelated limit) is 50%, being reduced to about 44% in the exact wavefunction32.
Support to our computational results is provided by the good agreement between calculated N-particle excitation energies and excitation energies extracted from B K-edge RIXS experiments3. This is apparent by comparing numerical (MRCI level of theory) and experimental values in the last two columns of Table 1 and Fig. 3(a). In other words, the numerically obtained excitation energies fit the measured excited-state relative energies; this good agreement allows for a one-to-one assignment of the main features for less than ~4 eV energy loss in the RIXS spectra. But the interpretation of the electronic ground state itself and of the lower N-particle charge excitations resulting from this assignment is peculiar, with no correspondence in the extensive literature available on MB6 hexaborides.
A series of experiments was conducted for a CaB6 and b YbB6, involving angle-resolved photoemission spectroscopy (ARPES), surface x-ray photoemission (SXE), resonant-inelastic x-ray scattering (RIXS), and partial fluorescence-yield (PFY) spectroscopy (in black). Multi-reference configuration interaction (MRCI) energies are given for comparison (in red) and all energies are referenced onto the Fermi energy EF. Figure 3(a) reprinted with permission from Denlinger et al.3, copyright (2022) by the American Physical Society, and Fig. 3(b) reprinted with permission from Denlinger et al.20, copyright (2022) by World Scientific Publishing Co. PTE. LTD. through the Copyright Clearance Center (CCC).
That computations beyond the CAS(6,6) level are necessary is visible from the large corrections brought through the MRCI treatment to some of the excitation energies, in particular, to the relative energies of singlet and triplet states at 2.4–3.5 eV and of the 7A1u septet, see Table 1. These corrections amount to up to 1.5 eV and point to configuration-interaction (CI) effects that are not yet captured with a six-orbital active-space CASSCF. This is evident, e. g., from the substantial reduction of the weight of the leading configuration in the CI wavefunction of each of those states. Moreover, we found that more approximate post-CASSCF CI schemes such as spectroscopy-oriented CI (SORCI) or second-order perturbation theory (PT2) treatments such as N-electron valence PT2 correction (NEVPT2) yield inaccurate excitation energies (see Supplementary Table 1). Relative shifts of this magnitude when improving the description of electronic correlations (by appropriately enlarging the active orbital space in CASSCF and/or post-CASSCF correlation treatment) are typically found in d-electron oxides, e.g., for d6 states in LaCoO333.
Correlated electronic structure of YbB6
For comparison, similar quantum chemical computations were carried out for the N-particle B62− electronic structure in the rare-earth hexaboride YbB6. The relative energies of the first twelve excited states and leading electronic configurations are listed in Table 2. In agreement with the computational results obtained for CaB6, the ground-state wavefunction is of strong multi-configurational character, with its leading configuration amounting to only 60%. Also in this case, the calculated MRCI energies can be compared with B K-edge X-ray spectra; MRCI N-particle excitation energies are overlaid for this purpose onto the experimental plots of Denlinger et al.20 in Fig. 3. It is seen that the relative energy of the well-defined first peak is rather well reproduced in both compounds. At higher energies, the level structure is denser; qualitatively, there is reasonably good correspondence between the peak found experimentally in each of the two materials at 2–3 eV and the excitations computed in that energy range.
The B p-p excitation energies computed for YbB6 are slightly lower as compared to CaB6. The likely explanation is that the larger effective charge of Yb ions in YbB6 (2 or slightly larger than 2 according to experimental estimates17,34,35,36, a representation that is also adopted for the surroundings of the B6 octahedron in our calculations) stabilizes the t1u B-cluster orbitals as compared to the eg (also a1g) components and reduces this way the eg → t1u excitation energies—the t1u composites have stronger anti-bonding character, are more extended, and therefore sense more effectively the nearest-neighbor cations.
Conclusions
While strong correlation effects within a whole manifold of electron configurations were already pointed out for the case of d-ion clusters37,38,39,40,41 we here describe similar physics occurring on p-electron octahedral clusters as found in MB6 hexaborides. In particular, we highlight the role of correlation effects on B6 octahedral units. For divalent CaB6 and YbB6 our quantum-chemically computed valence-shell excitation energies agree well with peak positions in earlier B K-edge RIXS measurements3,20, providing an interpretation of the RIXS spectrum. Shifts to lower energies of the N-particle excited states in YbB6 are ascribed to a relative stabilization of the anti-bonding t1u cluster orbitals as a result of larger cation charges in the Yb compound. Given (i) the limitations of density functional theory in describing the electronic structure of hexaboride compounds, documented in the case of CaB6 in literature extending over a few decades3,10,11,12,13,24, and (ii) more recent controversies on the electronic structures of e. g. SmB69,18,19 and YbB615,16,17, an extension of the present investigation of N-particle excitations to quasi-particle band structures is highly desirable. The foundations of quasi-particle band-structure calculations with ab initio wavefunction-based methods were laid22,23,42 and such an approach provides better control over the underlying approximations as compared to mainstream computational schemes based on density functional theory. The failures of the latter in hexaborides appear to be related to the correlated nature of the B62− electronic wavefunctions.
Methods
As material model, a B62− cluster was embedded in a field of M (with M = Ca, Yb) and B point charges (see Fig. 1 and Supplementary Table 2 for the cluster coordinates). The point charge field was created using the Ewald program43,44. For the lattice parameters, experimental data were used: lattice vectors a = 4.1537 Å and an x-axis B atom position xB = 0.201 for CaB645,46,47 and parameters of a = 4.1792 Å and xB = 0.202 for YbB617,48. To account for covalency effects, an initial charge value of +1.8 e was chosen for Ca ions in the embedding, which corresponds to an ionic charge of −0.3 e for B. In contrast, in YbB6, initial effective charges of +2.0 e for Yb17,34,35,36 and −0.33 e for B were assumed. For both embeddings, these charges were slightly adjusted by Ewald to ensure convergence of the Madelung sum. The final point charge values do not deviate more than 0.01 e from the initial values. Charge neutrality was ensured in the overall system by slightly adjusting a set of about 150 B point charges at the boundary of the whole array of approximately 40 000 point charges. To eight M nearest neighbors and to six adjacent B6 octahedra around the reference B62− quantum cluster (see Fig. 1) we assigned capped effective core potentials (cECPs); the pseudopotentials of Kaupp et al.49, Dolg et al.50. and Bergner et al.51. were employed for Ca, Yb and B, respectively. Their main purpose is to prevent electron density from the quantum cluster being artificially polarized by nearby point charges. Consequently, for the quantum cluster, an all-electron correlation-consistent polarized valence quadruple zeta quality (cc-pVQZ) basis set52 was used in conjunction with the CASSCF method described above.
Data availability
Supplementary Data (excitation energies of CaB6 using further methods, cluster and point charge field geometries) is attached and Orca calculations outputs are deposited at the ioChem-BD repository under the https://doi.org/10.19061/iochem-bd-6-146. Further datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Inosov, D. Rare-Earth Borides https://doi.org/10.1201/9781003146483 (Jenny Stanford Publishing, 2021, ISBN 9789814877565).
Cahill, J. T. & Graeve, O. A. Hexaborides: a review of structure, synthesis and processing. J. Mater. Res. Technol. 8, 6321–6335 (2019).
Denlinger, J. D. et al. Bulk band gaps in divalent hexaborides. Phys. Rev. Lett. 89, 157601 (2002).
Lortz, R. et al. Superconductivity mediated by a soft phonon mode: Specific heat, resistivity, thermal expansion, and magnetization of YB6. Phys. Rev. B 73, 024512 (2006).
Swanson, L. W., Gesley, M. A. & Davis, P. R. Crystallographic dependence of the work function and volatility of LaB6. Surf. Sci. 107, 263 (1981).
Gavilano, J. L. et al. Low temperature nuclear magnetic resonance studies of EuB6. Phys. Rev. Lett. 81, 25 (1998).
Cameron, A. S., Friemel, G. & Inosov, D. S. Multipolar phases and magnetically hidden order: review of the heavy-fermion compound Ce1−xLaxB6. Rep. Prog. Phys. 79, 066502 (2016).
Thalmeier, P., Akbari, A. & Shiina, R. Multipolar order and excitations in rare-earth boride Kondo systems. In Rare-Earth Borides (ed. Inosov, D.) Ch. 8 (Jenny Stanford Publishing, 2021).
Li, L., Sun, K., Kurdak, C. & Allen, J. W. Emergent mystery in the Kondo insulator samarium hexaboride. Nat. Rev. Phys. 2, 463–479 (2020).
Hasegawa, A. & Yanase, A. Electronic structure of CaB6. J. Phys. C: Solid State Phys. 12, 5431 (1979).
Massidda, S., Continenza, A., Pascale, T. M. D. & Monnier, R. Electronic structure of divalent hexaborides. Z. Phys. B 102, 83–89 (1997).
Rodriguez, C. O., Weht, R. & Pickett, W. E. Electronic fine structure in the electron-hole plasma in SrB6. Phys. Rev. Lett. 84, 3903 (2000).
Kino, H., Aryasetiawan, F., Terakura, K. & Miyake, T. Abnormal quasiparticle shifts in CaB6. Phys. Rev. B 66, 121103(R) (2002).
Kino, H. et al. GW quasiparticle band structure of CaB6. J. Phys. Chem. Solids 63, 1595–1597 (2002).
Neupane, M. et al. Non-Kondo-like electronic structure in the correlated rare-earth hexaboride YbB6. Phys. Rev. Lett. 114, 016403 (2015).
Zhang, T. et al. Electronic structure of correlated topological insulator candidate YbB6 studied by photoemission and quantum oscillation. Chin. Phys. B 29, 017304 (2020).
Kang, C.-J. et al. Electronic structure of YbB6: Is it a topological insulator or not? Phys. Rev. Lett. 116, 116401 (2016).
Sundermann, M. et al. 4 f crystal field ground state of the strongly correlated topological insulator SmB6. Phys. Rev. Lett. 120, 016402 (2018).
Amorese, A. et al. Resonant inelastic x-ray scattering investigation of the crystal-field splitting of Sm3+ in SmB6. Phys. Rev. B 100, 241107(R) (2019).
Denlinger, J. D., Gweon, G.-H., Allen, J. W., Bianchi, A. D. & Fisk, Z. Bulk band gaps in divalent hexaborides: A soft x-ray emission study. Surf. Rev. Lett. 09, 1309–1313 (2002).
Hozoi, L., Laad, M. S. & Fulde, P. Fermiology of cuprates from first principles: From small pockets to the Luttinger Fermi surface. Phys. Rev. B 78, 165107 (2008).
Hozoi, L., Birkenheuer, U., Fulde, P., Mitrushchenkov, A. O. & Stoll, H. Ab initio wave function-based methods for excited states in solids: correlation corrections to the band structure of ionic oxides. Phys. Rev. B 76, 085109 (2007).
Stoyanova, A., Mitrushchenkov, A. O., Hozoi, L., Stoll, H. & Fulde, P. Electron correlation effects in diamond: A wave-function quantum-chemistry study of the quasiparticle band structure. Phys. Rev. B 89, 235121 (2014).
Tromp, H. J., van Gelderen, P., Kelly, P. J., Brocks, G. & Bobbert, P. A. CaB6: A new semiconducting material for spin electronics. Phys. Rev. Lett. 87, 016401 (2001).
Helgaker, T., Jorgensen, P. & Olsen, J. Molecular Electronic-Structure Theory (Wiley VCH, Chichester, 2000).
Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. e1606 https://doi.org/10.1002/wcms.1606 (2022).
Janssen, G. J. M. & Nieuwpoort, W. C. Band gap in NiO: A cluster study. Phys. Rev. B 38, 3449 (1988).
Martin, R. L. Cluster studies of La2CuO4: A mapping onto the Pariser-Parr-Pople (PPP) model. J. Chem. Phys. 98, 8691 (1993).
Bogdanov, N. A., Manni, G. L., Sharma, S., Gunnarsson, O. & Alavi, A. Enhancement of superexchange due to synergetic breathing and hopping in corner-sharing cuprates. Nat. Phys. 18, 190 (2022).
Wehling, T. O. et al. Strength of effective Coulomb interactions in graphene and graphite. Phys. Rev. Lett. 106, 236805 (2011).
Craco, L., da Silva Pereira, T. A. & Leoni, S. Electronic structure and thermoelectric transport of black phosphorus. Phys. Rev. B 96, 075118 (2017).
Jensen, F. Introduction to Computational Chemistry, 2nd ed. (Wiley & Sons Ltd., 2007).
Hozoi, L., Birkenheuer, U., Stoll, H. & Fulde, P. Spin-state transition and spin-polaron physics in cobalt oxide perovskites: ab initio approach based on quantum chemical methods. N. J. Phys. 11, 023023 (2009).
Nanba, T. et al. Valency of YbB6. Phys. B Condens. Matter 186-188, 557–559 (1993).
Gavilano, J. et al. NMR studies of YbB6. Phys. B: Condens. Matter 329-333, 570 (2003).
Zhou, Y. et al. Pressure-induced quantum phase transitions in a YbB6 single crystal. Phys. Rev. B 92, 241118 (2015).
Majumdar, D. & Balasubramanian, K. Theoretical study of the electronic states of Nb4, Nb5 clusters and their anions (Nb4−, Nb5−). J. Chem. Phys. 121, 4014 (2004).
Sharma, S., Sivalingam, K., Neese, F. & Chan, G. K.-L. Low-energy spectrum of iron–sulfur clusters directly from many-particle quantum mechanics. Nat. Chem. 6, 927–933 (2014).
Presti, D., Stoneburner, S. J., Truhlar, D. G. & Gagliardi, L. Full correlation in a multiconfigurational study of bimetallic clusters: Restricted active space pair-density functional theory study of [2Fe–2 S] systems. J. Phys. Chem. C. 123, 11899–11907 (2019).
Hozoi, L., Eldeeb, M. S. & Rößler, U. K. V4 tetrahedral units in AV4X8 lacunar spinels: Near degeneracy, charge fluctuations, and configurational mixing within a valence space of up to 21 d orbitals. Phys. Rev. Res. 2, 022017 (2020).
Petersen, T. et al. How correlations and Spin–Orbit coupling work within extended orbitals of transition-metal tetrahedra of 4d/5d Lacunar Spinels. J. Phys. Chem. Lett. 13, 1681–1686 (2022).
Fulde, P. Wavefunctions of macroscopic electron systems. J. Chem. Phys. 150, 030901 (2019).
Derenzo, S. E., Klintenberg, M. K. & Weber, M. J. Determining point charge arrays that produce accurate ionic crystal fields for atomic cluster calculations. J. Chem. Phys. 112, 2074 (2000).
Klintenberg, M., Derenzo, S. & Weber, M. Accurate crystal fields for embedded cluster calculations. Comp. Phys. Commun. 131, 120–128 (2000).
Schmitt, K., Stückl, C., Ripplinger, H. & Albert, B. Crystal and electronic structure of BaB6 in comparison with CaB6 and molecular [B6H6]2−. Solid State Sci. 3, 321–327 (2001).
Lee, B. & Wang, L.-W. Electronic structure of calcium hexaborides. Appl. Phys. Lett. 87, 262509 (2005).
Schmidt, K. M., Buettner, A. B., Graeve, O. A. & Vasquez, V. R. Interatomic pair potentials from DFT and molecular dynamics for Ca, Ba, and Sr hexaborides. J. Mater. Chem. C. 3, 8649–8658 (2015).
Jun, J., Jiang, B. & Lemin, L. Study on band structure of YbB6 and analysis of its optical conductivity spectrum. J. Rare Earth 25, 654–664 (2007).
Kaupp, M., Schleyer, P. V. R., Stoll, H. & Preuss, H. Pseudopotential approaches to Ca, Sr, and Ba hydrides. Why are some alkaline earth MX2 compounds bent? J. Chem. Phys. 94, 1360 (1991).
Dolg, M., Stoll, H. & Preuss, H. Energy-adjusted ab initio pseudopotentials for the rare earth elements. J. Chem. Phys. 90, 1730 (1989).
Bergner, A., Dolg, M., Küchle, W., Stoll, H. & Preus, H. Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol. Phys. 80, 1431–1441 (1993).
Dunning, T. H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007 (1989).
Acknowledgements
We thank J.G., L.C., V.B., and P.F. for discussions, U.N. for technical assistance, and the German Research Foundation for financial support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
T.P. and L.H. carried out the quantum chemical calculations. U.K.R. and L.H. planned the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Physics thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Petersen, T., Rößler, U.K. & Hozoi, L. Quantum chemical insights into hexaboride electronic structures: correlations within the boron p-orbital subsystem. Commun Phys 5, 214 (2022). https://doi.org/10.1038/s42005-022-00979-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42005-022-00979-z
This article is cited by
-
Resonating holes vs molecular spin-orbit coupled states in group-5 lacunar spinels
Nature Communications (2023)
-
Dressed jeff-1/2 objects in mixed-valence lacunar spinel molybdates
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.