Abstract
Circadian clock proteins often reveal temperature-compensatory responses that counteract temperature influences to keep their enzymatic activities constant over a physiological range of temperature. This temperature-compensating ability at the reaction level is likely crucial for circadian clock systems, to which the clock proteins are incorporated, to achieve the system-level temperature compensation of the oscillation frequency. Nevertheless, temperature compensation is yet a puzzling phenomenon, since side chains that make up the clock proteins fluctuate more frequently due to greater thermal energy at higher temperature. Here, we investigated temperature influences on the dynamics of KaiC, a temperature-compensated enzyme (ATPase) that hydrolyzes ATP into ADP in the cyanobacterial circadian clock system, using quasielastic neutron scattering. The frequency of picosecond to sub-nanosecond incoherent local motions in KaiC was accelerated by a factor of only 1.2 by increasing the temperature by 10 °C. This temperature insensitivity of the local motions was not necessarily unique to KaiC, but confirmed also for a series of temperature-sensitive mutants of KaiC and proteins other than clock-related proteins. Rather, the dynamics associated with the temperature-compensatory nature of the reaction- and system-level was found in global diffusional motions, which was suggested to regulate the temperature dependence of ATPase activity and dephosphorylation process presumably through changes in the hexamer conformation of KaiC. The spatiotemporal scale at which cross-scale causality of the temperature sensitivity is established is finite, and extends down to picosecond to sub-nanosecond dynamics only in a very limited part of KaiC, not in its entire part.
Similar content being viewed by others
Introduction
Circadian clocks are endogenous timing systems that rhythmically control various biological processes with an ~24-h period1. This rhythm persists stably even without any external cues, and the period length is kept constant even when ambient temperature changes (temperature compensation). The phase of the clock system can be shifted upon receiving external stimuli such as light and temperature and then synchronized to the phase of the outer world. These unique characteristics enable organisms to optimize their fitness during day/night environmental cycles2,3,4,5.
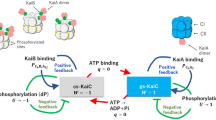
Temperature compensation is a remarkable characteristic of the circadian clock systems. Q10 values, the factor by which reaction speed or cycle frequency is accelerated by increasing the ambient temperature by 10 °C, are mostly in the range of 0.9–1.1 for circadian clock systems, whereas those of most biochemical reactions6 and the Belousov–Zhabotinsky oscillator7,8,9,10 range from 2 to 3. As schematically shown in Fig. 1a, circadian clocks exhibiting system-level temperature compensation often comprise unique clock proteins with temperature-compensated biochemical activities such as adenosine triphosphate (ATP) hydrolysis (ATPase)11,12,13, and protein phosphorylation (kinase)14,15,16 and dephosphorylation (phosphatase)14,15. A simple but attractive idea that emerged from studies12,13,15,17,18 of these biochemical activities is that the reaction-level temperature compensability is somehow correlated to system- or even higher cell-level temperature compensability (Fig. 1a). A great deal of effort has been devoted to elucidating the mechanism of this connectivity via experimental12,13,15,16,18 and modeling19,20,21,22,23 approaches.
a Spatiotemporal hierarchy spanning from atomic-scale dynamics, molecular-scale reaction, molecular system-scale frequency, and cellular-scale frequency. When the ATPase activity of KaiC doubles as a result of amino acid substitutions (orange arrow), both the in vitro system- and cell-level frequencies also double12,17,25. In cyanobacteria, the temperature (T) sensitivity of ATPase is correlated with those of the system and cell levels. (Inset) Three extreme cases of temperature influence on clock-protein dynamics. Thermal fluctuations are sensitively accelerated, insensitively compensated, or sensitively compensated through a balance of opposing contributions. b Temperature-dependent ATPase mutation sites mapped onto the crystal structure of KaiC32,33. Zoomed-in-views of c S258, d F247, e Q361, f N390, g F470, and h phosphorylated S431 and T432 in KaiC. Hydrogen bonds are highlighted by green dotted lines.
Nevertheless, temperature compensation remains a puzzling phenomenon in terms of protein dynamics, as atoms and side chains in proteins should fluctuate more frequently at higher temperature due to their greater thermal energy. Three extreme cases can be considered (inset in Fig. 1a). First, thermal fluctuation in these key clock proteins is accelerated in a temperature-dependent manner, as observed for ordinary proteins. Second, the atoms and side chains in temperature-compensated clock proteins fluctuate in a temperature-insensitive manner via unknown mechanisms. Third, some opposing but balancing contributions act at the level of protein dynamics level, i.e., one contribution accelerates but the other decelerates so that they differentially affect the elementary reaction steps to achieve overall compensation. Experimental investigations of the dynamics of temperature-compensated clock proteins are needed to distinguish among these cases.
KaiC, a temperature-compensated clock protein in cyanobacterium Synechococcus elongatus PCC 794211,12,13, acts as a circadian oscillator with other two clock proteins, KaiA and KaiB24. Its most striking feature is that its temperature-compensated circadian rhythm can be reconstructed even in vitro by mixing KaiA, KaiB, and KaiC in the presence of ATP25 (Fig. 1a). An ATP molecule binds a Walker motif in each of the tandemly duplicated domains called the N-terminal C1 and C-terminal C2 domains (Fig. 1b). These ATP binding events trigger oligomerization of KaiC into a double-ring hexamer. The ATP molecule bound to the C2 domain (C2-ATP) is used mostly as the source of phosphoryl group that is transferred to (auto-kinase) and then removed from (auto-phosphatase) S431 and T432 in KaiC. In the presence of KaiA and KaiB, the status of the dual phosphorylation site alters in a cyclic manner: ST → SpT → pSpT → pST → ST, where S, T, pS, and pT represent S431, T432, phosphorylated S431, and phosphorylated T432, respectively26,27. The frequency of this phosphorylation cycle (P-cycle) is proportional to the rate of hydrolysis of C1-bound ATP (C1-ATP) into adenosine diphosphate (ADP) in the absence of KaiA and KaiB (Fig. 1a)12,13,17. More importantly, the ATPase activity of KaiC is perfectly temperature-compensated (Q10ATP = 1.0)12. The in vitro Kai oscillator that consists of temperature-compensated KaiC provides a practical means for studying cross-scale properties of temperature-compensation phenomena at the system, reaction, and dynamics levels (Fig. 1a).
Quasielastic neutron scattering (QENS) is a powerful and direct technique for accessing protein dynamics at the picosecond to nanosecond time scales28. Because hydrogen atoms, which constitute up to half of all atoms in proteins, are distributed near uniformly in the three-dimensional structures of proteins, an averaged view of protein dynamics can be extracted from QENS spectra29. In this study, we designed a series of KaiC mutants (Fig. 1b) with temperature-dependent periods (Fig. 1c–e), inverse temperature-dependent period (Fig. 1f), temperature-compensated but shortened-period (Fig. 1g), or arrhythmicity (Fig. 1h), and measured the temperature sensitivity of their protein dynamics using the neutron spectrometer BL02 (DNA: Biomolecular Dynamics Spectrometer) in the Material and Life Science Experimental Facility of Japan Proton Accelerator Research Complex (MLF/J-PARC)30. Our QENS data revealed that the frequency of picosecond to sub-nanosecond incoherent motions is essentially temperature-insensitive in KaiC, regardless of the presence or absence of the temperature-sensitive mutations. The spatiotemporal scale at which cross-scale causality of the temperature sensitivity is established is finite, and extends down to picosecond to sub-nanosecond dynamics only in a very limited part of KaiC, not in its entire part.
Results
Screening and characterization of temperature-sensitive mutants of KaiC
As confirmed by the positions along the horizontal axis in Fig. 2a, the ATPase activity of KaiCWT is as low as 11 d−1 and almost temperature-insensitive (Q10ATPWT = 0.89 ± 0.10), as previously reported12. Consistently with previous studies11,15,25, the P-cycle frequency (fP = 24/period) of KaiCWT in the presence of KaiA and KaiB was also temperature-compensated (Fig. 2b) (Q10fpWT = 1.08 ± 0.04). Consequently, data points taken at different temperatures for KaiCWT nearly overlapped in the ATPase–fP plot (Fig. 2a). Taking advantage of potential reaction–system correlations, we screened the temperature-sensitive mutants of KaiC using an in vitro ATPase-based screening system31. Among a number of KaiC mutants screened for temperature-dependent ATPase activity, four candidates were characterized in detail as they revealed stable but temperature-dependent system-level oscillation (Fig. 2c–f).
a Temperature dependence of the relationship between the ATPase activity of KaiC alone and in vitro KaiC phosphorylation-cycle (P-cycle) frequency (fP = 24/period) in the presence of KaiA and KaiB. P-cycles of b KaiCWT, c KaiCS258A, d KaiCF247A, e KaiCQ361E, f KaiCN390A, g KaiCF470Y at four different temperatures: 303 (blue), 308 (green), 313 (orange), and 318 K (red). Dark-colored circles correspond to the mean from independent preparations and measurements (pale-colored squares, triangles, and diamonds). Light blue circles plotted in panel b correspond to the P-cycle of KaiCWT in a D2O buffer. h Correlation of Q10 values between ATPase (Q10ATP) and P-cycle (Q10fp). Error bars correspond to one standard deviation obtained from three independent experiments, except that they are shown as three standard deviations for Q10fp.
The first was the S258A mutant of KaiC (KaiCS258A). According to the X-ray crystal structure of KaiCWT32,33, S258 is located in the C2 domain (Fig. 1b and c). The ATPase activity of KaiCS258A at 303 K (6.3 ± 1.1 d−1) was lower than that of KaiCWT (11.8 ± 1.1 d−1) but increased in a temperature-dependent manner up to 14.1 ± 1.8 d−1 at 318 K (Q10ATPS258A = 1.73 ± 0.22) (Fig. 2a). Consistent with this, the fP value for KaiCS258A increased from 0.78 to 1.40 d−1 as the temperature increased (Fig. 2c) (Q10fpS258A = 1.56 ± 0.01). Because of this correlation, the data trace for KaiCS258A extends almost diagonally from low- to high-temperature conditions in the ATPase–fP plot (Fig. 2a). The second and third mutants (KaiCF247A, KaiCQ361E), which replaced residues that neighbored in the C1–C2 interface (Figs. 1b, 2d, and e), are also traced diagonally (Fig. 2a). The fP value for KaiCF247A increased from 1.12 to 1.97 d−1 as temperature increased (Fig. 2d) (Q10fpF247A = 1.63 ± 0.01), as observed for the mutant’s ATPase activity (Fig. 2a) (Q10ATPF247A = 1.47 ± 0.05), whereas KaiCQ361E exhibited a slightly weakened correlation between Q10ATPQ361E (1.70 ± 0.15) and Q10fpQ361E (1.23 ± 0.02) (Fig. 2e and h). The fourth is an inverse temperature-dependent mutant that harbors the N390A substitution in the C2–C2 interface (KaiCN390A, Fig. 1f) nearby the C1 domain (Fig. 1b). The ATPase activity and fP value of KaiCN390A became smaller at higher temperatures (Fig. 2f) in a correlated manner, as evidenced by its diagonal trace (Fig. 2a) with inversed temperature sensitivities (Q10ATPN390A = 0.47 ± 0.07, Q10fpN390A = 0.69 ± 0.02). However, KaiCN390A became arrhythmic at the highest temperature of 318 K (Fig. 2f).
Taking into consideration the limited persistence of the P-cycle for KaiCF247A and KaiCN390A at 318 K (Fig. 2d and f), we dissolved KaiCWT and the four KaiC mutants exhibiting reaction–system correlation in a D2O buffer (Supplementary Note 1) and subjected them to QENS experiments at different temperatures: 283, 293, 302, 313, and 317 K for KaiCWT, 302 (low) and 310 or 313 K (high) for KaiCS258A, KaiCF247A, KaiCQ361E, and KaiCN390A. For comparison purposes, we conducted further QENS experiments using two other KaiC mutants12,25; a temperature-compensated but period-shortened F470Y mutant of KaiC (KaiCF470Y: Q10ATPF470Y = 1.07 ± 0.06 and Q10fpF470Y = 1.09 ± 0.05) (Figs. 1b, 1g, 2a, 2g, and 2h) and a temperature-compensated but arrhythmic S431D/T432E double mutant (KaiCDE: Q10ATPDE = 0.98 ± 0.07) mimicking the fully phosphorylated KaiC-pSpT (Figs. 1b, 1h, 2a, and 2h). Noted that the circadian rhythm of KaiCWT was barely influenced by the D2O buffer used in this study (light blue circles in Fig. 2b).
KaiC dynamics detected by QENS
To investigate the temperature dependence of KaiC dynamics, we conducted QENS experiments of KaiCWT at five different temperatures from 283 to 317 K. It must be noted that 293 K is approximately the critical temperature of the in vitro oscillator34, below which the P-cycle becomes arrhythmic (zero amplitude). As shown in Fig. 3a, a difference QENS spectrum S(Q, E) of KaiCWT, where Q is the momentum transfer and E is the energy transfer of neutrons, could be obtained at each temperature by subtracting the background spectrum of the D2O solvent from the sample spectrum, on the basis of scaling factors calculated from neutron scattering cross-sections. The resultant S(Q, E) for KaiCWT at all the temperatures shared a common feature of temperature-dependent broadening of a narrow elastic peak and a wide quasielastic component derived from global and local motions, respectively (Fig. 3a). To analyze the Q- and temperature dependencies of these two components quantitatively, we attempted to fit the following equation28 to each S(Q,E) in Fig. 3b–f:
where A0(Q) is the elastic incoherent structure factor (EISF); δ(E) is Dirac delta function; RF(Q,E) is the instrumental resolution function obtained from the spectrum of vanadium; BG(Q) is the background; and Llocal(Q,E) and Lglobal(Q,E) are Lorentzian functions, (Γ(Q)/π) (E2 + Γ(Q)2)−1, describing local internal motion and global diffusive motion of the protein, respectively, where Γ(Q) is the half-width at half maximum. Q-averaged χ2 values resulting from fitting of Eq. (1) to the experimental data of KaiCWT was ~0.93 ± 0.07 (Fig. 3b–f), assuring optimum quality of the curve-fitting procedure.
a S(Q,E) measured at five different temperatures from 283 to 317 K. Experimental spectra, Sexp(Q,E), at b 283 K, c 293 K, d 302 K, e 313 K, and f 317 K are fitted using Sfit(Q,E), which includes contributions of two Lorentzian functions, LZ1(Q,E) = Lglobal(Q,E) and LZ2(Q,E) = Lglobal(Q,E)\(\times\)Llocal(Q,E), background BG(Q), and resolution function RF(Q,E) as defined in Eq. (1). The error bars come from the propagation of the standard deviation of the Poisson distribution in creating Q–E maps.
For the KaiC mutants, QENS experiments were conducted at low (302 K) and high (310 or 313 K) temperatures that were within the physiologically functional range of temperature as the circadian clock. Although most of the KaiC mutants showed similar S(Q,E) to KaiCWT (Fig. 4a, 4c, 4e, and 4f), visually distinguishable differences were observed for KaiCF247A and KaiCN390A (Fig. 4b and d). In order to make more detailed and quantitative comparisons, each S(Q,E) of the KaiC mutants was analyzed using Eq. (1) in the same way as KaiCWT (panels g–r in Fig. 4). In the following, we describe the global and local motions of KaiC on the basis of temperature dependencies of Γglobal(Q) and Γlocal(Q), respectively.
Comparison of QENS spectra acquired at low and high temperatures for a KaiCS258A, b KaiCF247A, c KaiCQ361E, d KaiCN390A, e KaiCF470Y, and f KaiCDE. Blue, orange, and pink lines correspond to the spectra at 302, 313, and 310 K, respectively. For comparison, the QENS spectra of KaiCWT at 313 K (black thick line) are normalized to match the top-peak intensity of the mutants. g–l At the low temperature of 302 K. m–r At a high temperature of 310 or 313 K. Experimental spectra, Sexp(Q,E), are fitted using Sfit(Q,E), which includes contributions of two Lorentzian functions, LZ1(Q,E) = Lglobal(Q,E) and LZ2(Q,E) = Lglobal(Q,E)\(\times\)Llocal(Q,E), background BG(Q), and resolution function RF(Q,E) as defined in Eq. (1). The error bars come from the propagation of the standard deviation of the Poisson distribution in creating Q–E maps.
Global motions of KaiC
Γglobal(Q) provides information on the frequency of the global motions, whose properties are most straightforwardly inspected using the plot of Γglobal(Q) vs Q2. Linear Q2-dependencies of Γglobal(Q) were confirmed for KaiCWT at every temperature (Fig. 5a), indicating its boundary-free diffusions including translational and rotational diffusions. Thus, the linear slope in Fig. 5a relates to an apparent boundary-free diffusion coefficient (Dglobal). The Dglobal for KaiCWT at 302 and 313 K were 3.54 ± 0.09 and 4.48 ± 0.10 × 10−7 cm2 s−1, respectively (Fig. 5h), which were similar to those (3.75 and 4.97 × 10−7 cm2 s−1) calculated using the known crystal structure of the KaiC hexamer (Supplementary Note 2).
a KaiCWT, b KaiCS258A, c KaiCF247A, d KaiCQ361E, e KaiCN390A, f KaiCF470Y, and g KaiCDE. The slope of each linear fit corresponds to the apparent diffusion coefficient, Dglobal. Error bars correspond to the errors resulted from the curve-fitting analysis using Eq. (1) as shown in Figs. 3 and 4. h Dglobal and its temperature dependence, Q10global. White bars for KaiCS258A, KaiCF247A, and KaiCQ361E correspond to the Dglobal values at 313 K, which were estimated by linear extrapolation of the Dglobal values determined at 302 and 310 K. A hatched bar represents Q10globalWT estimated from the slope of Arrhenius plot using five different temperatures (283, 293, 302, 313, and 317 K) (Supplementary Fig. 2a), as previously reported65. A shaded bar represents Q10globalWT estimated from the slope of Arrhenius plot using three different temperatures (302, 313, and 317 K) within the physiologically functional range of temperature (Supplementary Fig. 2a). Other Q10global presented as black bars were estimated as the ratio of measurement results (RLow and RHigh) at two temperatures (TLow and THigh) using Q10 = (RHigh/RLow)10/(THigh-TLow); TLow = 302 K, THigh = 313 or 310 K. Error bars correspond to the errors associated with the linear regression analysis (a–g).
The temperature dependencies of the Dglobal (Q10global) for KaiCWT and the KaiC mutants (Fig. 5b–g) were ~1.2 (Fig. 5h), similar to other examples: ~1.2 (300–310 K) in human hemoglobin (Hb)35 and ~1.2 (290–300 K) in α-synuclein (αSyn)36. These observations are essentially consistent with the Stokes-Einstein diffusion that predicts Dglobal \(\propto\) T/η(T), where T and η(T) are the temperature and solvent viscosity at T, respectively. Assuming η(302) = 1.016 cP and η(313) = 0.799 cP for the present D2O solvent (Supplementary Note 2), the term of T/η(T) increases 1.3-fold by raising the temperature form 302 to 313 K. This agreement between Q10global and the increase ratio of T/η(T) indicates that the temperature dependence of the hydrodynamic radius is rather small. Thus, we suggest that KaiCWT and the KaiC mutants are maintained as intact hexamers during the QENS measurements in the observed temperature range.
In contrast to Q10global, the absolute Dglobal values of the temperature-sensitive mutants shared a common feature. The Dglobal values estimated at high temperature for KaiCS258A, KaiCF247A, KaiCQ361E, and KaiCN390A were systematically smaller than those of KaiCWT, KaiCF470Y, and KaiCDE (Fig. 5h). In order to make a fair comparison, it is necessary to take into account that the measured temperature of KaiCS258A, KaiCF247A, KaiCQ361E is lower by 3 K than the others. On the basis of the pseudo-linear temperature dependence of the Dglobal values for KaiCWT (Fig. 5h), the Dglobal values at 313 K for KaiCS258A, KaiCF247A, KaiCQ361E were estimated by linear extrapolation of the Dglobal values determined at 302 and 310 K (white bars in Fig. 5h). Even after applying these corrections, the Dglobal values tended to be smaller in the temperature-sensitive mutants. These results suggest that the global hexamer structure of the temperature-sensitive mutants may be slightly different from those of KaiCWT and the temperature-compensated KaiC mutants (KaiCF470Y, KaiCDE).
Local motions of KaiC
In contrast to Γglobal(Q), Γlocal(Q) reflects picosecond to sub-nanosecond incoherent local fluctuations of side-chain and main-chain in KaiC. Γlocal(Q) of KaiCWT increased asymptotically to approach a plateau at high Q2 (Fig. 6a). As at high Q, the motions over short distances predominate, the plateau values correspond to the elementary displacements of the local motions. Slight but temperature-dependent increase in the high-Q plateau of KaiCWT suggests that the side-chain and main-chain motions in KaiCWT are accelerated by thermal fluctuations (Fig. 6a).
a KaiCWT, b KaiCS258A, c KaiCF247A, d KaiCQ361E, e KaiCN390A, f KaiCF470Y, and g KaiCDE. Solid lines represent resultant fits using a jump-diffusion model that predicts the jump-diffusion coefficient, Dlocal, as the curvature of the saturating curves and the reciprocal of residence time, τ−1, as Γlocal(Q) converged at infinite Q. Error bars correspond to the errors resulted from the curve-fitting analysis using Eq. (1) as shown in Figs. 3 and 4. h τ−1 and its temperature dependence, Q10local. i Dlocal and its temperature dependence, Q10D-local. A hatched bar represents Q10localWT or Q10D-localWT estimated from the slope of Arrhenius plot using five different temperatures (283, 293, 302, 313, and 317 K) (Supplementary Fig. 2b and 2c), as previously reported65. A shaded bar represents Q10localWT or Q10D-localWT estimated from the slope of Arrhenius plot using three different temperatures (302, 313, and 317 K) within the physiologically functional range of temperature (Supplementary Fig. 2b and 2c). Other Q10local presented as black bars were estimated as the ratio of measurement results (RLow and RHigh) at two temperatures (TLow and THigh) using Q10 = (RHigh/RLow)10/(THigh-TLow); TLow = 302 K, THigh = 313 or 310 K. Error bars correspond to the errors associated with the analysis using jump-diffusion model28 (a–g).
To determine the temperature sensitivity of the local motions quantitatively, Q2-dependencies of Γlocal(Q) were analyzed by using a jump-diffusion model28: Γlocal(Q) = DlocalQ2 (1 + DlocalQ2τ)−1, where Dlocal is the jump-diffusion coefficient and τ is the residence time spent on one site before jumping to others. The model gave reasonable fits to temperature- and Q2-dependent Γlocal(Q) of KaiCWT (lines in Fig. 6a). The resultant τ−1 values for KaiCWT were slightly temperature-dependent (Fig. 6h). Arrhenius plot analyses using five different temperatures (283, 293, 302, 313, and 317 K) resulted in the activation energy of 4.0 ± 0.6 kcal mol−1 (Supplementary Note 3), which corresponded to the Q10 value of 1.23 ± 0.04 for the internal motions (Q10local, hatched bar in Fig. 6h). The estimation of the Q10localWT value was not seriously affected by limiting the physiologically functional range of temperature (302, 313, and 317 K: Q10localWT = 1.15 ± 0.11, shaded bar in Fig. 6h, Supplementary Note 3) and by taking a ratio of τ−1 at low (302 K) and high (313 K) temperatures (Q10localWT = 1.10 ± 0.14, black bar in Fig. 6h). The Q10 values of Dlocal (Q10D-local) for KaiCWT varied in the range from 1.16 to 1.36 depending on the analysis method (Fig. 6i), probably due to the larger associated errors compared to the τ−1 values.
Γlocal(Q) of the KaiC mutants, which revealed the same high-Q saturation as KaiCWT (Fig. 6b–g), could be analyzed reasonably with the jump-diffusion model. Regardless of whether they are temperature-compensated or temperature-dependent, the Q10local values of the KaiC mutants ranged from 0.97 to 1.20 and were comparable to KaiCWT within error (Fig. 6h). A similar trend was confirmed for Q10D-local (Fig. 6i). These results suggest that the average behavior of the local fluctuations occurring throughout KaiC, at least on picosecond to sub-nanosecond time scales, is not clearly correlated with the reaction and system levels in terms of temperature dependence.
While the mutations had small effects on the temperature dependence of the local dynamics, the absolute values of τ−1 were markedly different from KaiCWT in several temperature-sensitive mutants. The τ−1 values for KaiCF247A were 0.14 ± 0.01 ps−1 at low and high temperatures and were reduced ~50–70% relative to KaiCWT (Fig. 6h). Conversely, the local motions in KaiCN390A were 30–40% faster (0.29 ± 0.01–0.33 ± 0.02 ps−1) than KaiCWT (Fig. 6h). Dlocal was essentially unaffected by temperature change or mutations (Fig. 6i), except in the case of KaiCF247A, where a large error prevented us from identifying clear increase in Dlocal.
Fractional change in apparently immobile atoms in the temperature-dependent ATPase mutants of KaiC
EISF is defined as the ratio of the elastic peak intensity to the sum of elastic and quasielastic scattering intensities as in Eq. (1), providing information on the geometry of molecular motions and the fraction of mobile and immobile atoms on the time scale (~55 ps) of the spectrometer. As shown in Fig. 7a–g, EISF of KaiCWT and the mutants were plotted against Q and then fitted using the following equation, assuming diffusion in an ensemble of spheres whose radii (a) follow a lognormal distribution37:
where p is the fraction of atoms whose motions are outside the current instrumental energy window and therefore appear immobile; (1 − p) correspond to the fraction of atoms diffusing within the sphere ensemble; c is the median of the distribution; s is the variance in the natural logarithmic space; and j1 denotes the spherical Bessel function of the first kind of order.
a KaiCWT, b KaiCS258A, c KaiCF247A, d KaiCQ361E, e KaiCN390A, f KaiCF470Y, and g KaiCDE. Solid lines represent the resultant fits of Eq. (2). Error bars correspond to the errors resulted from the curve-fitting analysis using Eq. (1) as shown in Figs. 3 and 4. h Immobile fraction, p. i Radial distribution functions of mobile fraction. Black, purple, blue, pink, orange, and red lines correspond to the distributions at 283, 293, 302, 310, 313, and 317 K, respectively. Error bars correspond to the errors associated with the EISF analysis37 using Eq. (2) (a–g).
The results of EISF analysis support the current observations on the local dynamics. Consistent with the Q10localWT value of 1.15 ± 0.11, the immobile fraction of KaiCWT was maintained at ~0.6 in the physiologically functional range of temperature (Fig. 7h). In KaiCF470Y and KaiCDE, the immobile fractions were nearly unaffected by temperature change or mutations within the experimental error. On the other hand, the immobile fraction of KaiCF247A increased 25% as the temperature increased, despite its nearly temperature-compensated τ−1 value (Q10localF247A = 0.97 ± 0.16). Interestingly, a reverse trend was confirmed in KaiCN390A. At the same time, all the temperature-sensitive mutants of KaiC exhibited smaller immobile fractions than KaiCWT at 302 K. This result that the temperature-dependent mutations shifted certain motions from immobile to mobile fractions (Fig. 7h) is directly or indirectly related to the systematic decrease in the Dglobal values (Fig. 5h).
In KaiCWT and KaiCF470Y, atoms diffusing within a radius of 3–6 Å (Fig. 7i) constituted a major mobile component. By contrast, the mobile fractions of the temperature-sensitive mutants and KaiCDE were distributed mainly in reduced radii of 1–2 Å relative to KaiCWT. Thus, KaiCS258A, KaiCF247A, KaiCQ361E, and KaiCN390A are the temperature-dependent ATPase mutants with the reductions in Dglobal (Fig. 5h), immobile fraction p (Fig. 7h), and amplitudes of local motions (Fig. 7i).
Discussion
Over the past decades, chronobiologists have sought a reasonable model that explains the three physiological properties of the circadian clock systems: self-sustained oscillation, temperature compensation, and synchronization2,3,4. The circadian clock of cyanobacteria is an ideal experimental system for this purpose, as its physiological properties can be studied in relation to the physicochemical properties of clock-related components at the molecular and atomic scales.
Among the three Kai proteins, KaiC is the core of the cyanobacterial clock system. In the presence of both KaiA and KaiB, KaiC exhibits a phosphorylation rhythm (Fig. 2b) whose frequency (fP) is correlated with the ATPase activity of KaiC alone (Fig. 2a). For example, when the ATPase activity of KaiC doubles as a result of amino acid substitutions, the frequencies of both the in vitro system-scale and the cellular-scale rhythms also double (Fig. 1a)12,17,25.
This causal relationship, in which properties are transferred through upward causation from bottom to top in the spatiotemporal hierarchy, was also confirmed for temperature compensation from the reaction to the cellular scale (Fig. 1a). The ATPase activity of KaiCWT is temperature-compensated; probably because of this, both the in vitro and in vivo rhythms13 are also temperature-independent. This phenomenological interpretation is further supported by the four examples of temperature-sensitive mutants of KaiC. In contrast to KaiCWT (Q10ATPWT = 0.89 ± 0.10), the fP values for KaiCS258A (Q10ATPS258A = 1.73 ± 0.22), KaiCF247A (Q10ATPF247A = 1.47 ± 0.05), and KaiCQ361E (Q10ATPQ361E = 1.70 ± 0.15) increased in a temperature-dependent manner (Fig. 2c–e), and the fP value for KaiCN390A (Q10ATPN390A = 0.47 ± 0.07) obeyed the inverse temperature dependence (Fig. 2f). The well correlated Q10 values between the ATPase activity and fP values in the four mutants (Fig. 2h) clearly demonstrate that the reaction-to-system scale causal relationship arises from the temperature-compensated ATPase activity of KaiC.
In the present study, we utilized QENS technique to test how far down the microscopic spatiotemporal scale the causality of the temperature sensitivity can be established. The Q10global values were estimated to be ~1.2 for KaiCWT and the mutants (Fig. 5h), confirming that the global motions of KaiC are accelerated by the temperature dependence of T/η(T) according to the Stokes-Einstein diffusion law. The limited temperature dependence was also observed for the parameters, such as τ−1 and Dlocal, which characterize the local dynamics of KaiC. Even though the P-cycle and ATPase activity were accelerated in a temperature-dependent manner (Q10ATP and Q10fp as high as 1.7) in the three mutants (KaiCS258A, KaiCF247A, KaiCQ361E) and decelerated in the inverse temperature-dependent manner (Q10ATP and Q10fp as low as 0.5) in one mutant (KaiCN390A) (Fig. 2h), the Q10local and Q10D-local values for all the four temperature-sensitive mutants monotonously resulted in 1.0–1.2 (Fig. 6h and i). The similar temperature dependence was also confirmed for the local dynamics of the temperature-compensated but short-period (KaiCF470Y) and arrhythmic (KaiCDE) mutants. Our current QENS observations indicate that the spatiotemporal scale at which causality of the temperature sensitivity is established is finite. Given that QENS detects the average image of the fluctuations of all the hydrogen atoms scattered in the protein molecule, we can conclude that causality is established down to the local dynamics only in a very limited part of KaiC (inset in Fig. 1a).
It is worth discussing our observations in the light of previous QENS studies on proteins other than clock-related proteins. To date, the QENS method has been used in many studies to characterize protein dynamics of small single-domain proteins38,39 to larger and more complex molecular systems40,41,42,43, as well as to large-scale conformational changes such as those that occur upon ligand binding35,44,45, pressurization46,47,48, unfolding37,49,50,51,52,53, and fibrillization36,54. The results reported to date for various protein samples indicate that the τ values are mostly distributed in the range of 1–20 ps (τ−1 = 0.05–1.0 ps−1) (see Grimaldo et al29. and references therein), although attention must be paid to the differences in measurement temperature, energy resolution, and analysis methods. The τ value for KaiCWT, 3.7–8.0 ps (τ−1 = 0.13–0.27 ps−1), is included in the above range, indicating that the internal motion of KaiCWT is neither exceptionally slow nor too fast.
More detailed comparisons of internal motions are possible by making reference for QENS data for Hb35 and αSyn36, which were acquired at the same beamline with the same energy resolution (12 μeV/55 ps). As shown in Fig. 8, the temperature dependence of the jump-diffusion frequency of KaiCWT (Q10localWT = 1.1–1.2) is not substantially different from the four examples (Q10local = 1.1–1.3) for deoxygenated Hb (deoxy-Hb), CO-bound Hb (CO-Hb), a fibril state of αSyn (fib-αSyn), and a monomeric but intrinsically disordered form of αSyn (mon-αSyn). This result points to the intrinsically low energy barrier for the local motions independent of protein folds or functions, supporting the present interpretation that the temperature sensitivity of the Kai oscillator does not correlate well with that of the picosecond to sub-nanosecond local dynamics. In the measured range of temperature, however, the τ−1 values for KaiCWT are 30–50% smaller than those of the four examples. Considering the insensitivity of the internal dynamics even upon an R(CO)-to-T(deoxy) allosteric transition of Hb (Fig. 8), we speculate that the slower jump-diffusion frequency of KaiCWT may be related to its function and structure as the circadian pacemaker. In fact, the τ−1 values for KaiCF247A and KaiCN390A were 50–70% reduced and 30–40% enhanced, respectively, relative to KaiCWT (Figs. 6h and 8), although not for all temperature-sensitive mutants.
Dashed and solid red lines represent the linear fits of the Arrhenius plot for KaiCWT using entire (283–317 K) and physiologically functional range (302–317 K) of temperature, respectively. Quasielastic neutron scattering (QENS) data of Hb and αSyn are taken from previous studies35,36; activation energies of deoxygenated Hb (deoxy-Hb), CO-bound Hb (CO-Hb), fibrillized αSyn (fib-αSyn), and monomeric αSyn (mon-αSyn) are 5.2 ± 0.3, 5.2 ± 0.3, 3.7 ± 0.7, and 2.6 ± 1.9 kcal mol−1, respectively. Values given near fitting lines represent the Q10local values estimated for 303 K.
In contrast to the featureless temperature dependence of the local motions, all the temperature-sensitive mutants shared the reduced global motions including the translational and rotational diffusions relative to KaiCWT and temperature-insensitive mutants (KaiCF470Y and KaiCDE) (Fig. 5h). Two possibilities can be considered for the reduced Dglobal.
The first is the deformation into less compact hexamers by the temperature-sensitive mutations. According to the crystallographic structure of KaiC32,33, every temperature-sensitive mutation is mapped onto its domain-domain interfaces. S258 is located in the outer radius side of the C2 ring (Fig. 1b); the side chain is hydrogen bonded to that of R326 in the neighboring C2 domain (Fig. 1c). This inter–C2-domain hydrogen bond is thus disrupted in KaiCS258A. F247 and Q361 neighbor each other in the C1–C2 interface (Fig. 1b), which is stabilized by a limited number of inter-domain contacts with lower packing density than the C1–C1 and C2–C2 interfaces (Supplementary Note 4), exemplified by the non-bonded interactions associated with F247 and Q361 (Fig. 1b). The plane of the phenyl ring of F247 in the C1 domain lies nearly parallel to the C1–C2 interface (Fig. 1d), filling the loosely packed boundary through a hydrophobic interaction with L360 in the C2 domain. The F247A substitution is thus interpreted as the mutation that makes the intrinsically low-density interface even looser. Although the C1–C2 packing density will be little affected by the replacement of glutamine to glutamate in KaiCQ361E (Fig. 1b), a potential hydrogen bond between Q361 and either T238 or N245 in the C1 domain should be modulated or even disrupted (Fig. 1e). The N390A substitution disrupts an inter–C2-domain hydrogen bond between the side chain of N390 and the main-chain carbonyl oxygen atom of G386 (Fig. 1f). Considering these structural features common to the temperature-sensitive mutations, we speculate that the looser inter-domain interactions have resulted in the loss of compactness of the hexamer structure.
The second is the reduction of an additional contribution from internal large-scale motions. Previous QENS study on the R(CO)-to-T(deoxy) allosteric transition of Hb shows a certain similarity to our results35; Dglobal is notably increased upon the T(deoxy)-to-R(CO) transition while keeping τ−1 nearly unaffected. Authors of that study suggested that internal large-scale relative motions of two αβ dimers could partially contribute to Dglobal in CO-Hb35. If this is the case, the decrease in Dglobal is interpreted as a result of the suppression of domains movements within the KaiC hexamer by the temperature-sensitive mutations. In fact, several lines of evidence have pointed out the dynamic properties of inter-ring stacking of KaiC during the circadian cycle55.
Since the current temperature-sensitive mutations will not make the inter-domain interactions within the KaiC hexamer more stable, we believe that the contribution of the first case is greater than that of the second. This interpretation is also consistent with the decrease in the immobile fraction of the temperature-sensitive mutations (Fig. 7h). As shown in Fig. 2c–f, the temperature-sensitive mutations resulted in a preferential shortening and elongation of the dephosphorylation process in the P-cycle. The dephosphorylation process is also the timing of the ATPase activation in KaiC12. Taken together, these results suggest that delicate but close inter-domain interactions are important in regulating the temperature dependence of ATPase and dephosphorylation reactions in KaiC. This view is also supported by the fact that the KaiC hexamer undergoes a quaternary structure change in concert with ATPase and dephosphorylation events33.
Several studies have suggested that fluctuations and structural polymorphs of the clock proteins play important roles in the circadian clock systems. In mammalian systems, CKIδ-dependent phosphorylation is one of the key reactions that regulates period length and its temperature sensitivity16. In CKIδ, the temperature dependence of substrate affinity is compensated by the opposing temperature dependence of product affinity18. On the basis of molecular dynamics (MD) simulation, the authors of that study proposed that the temperature dependence of the amplitude of particular local fluctuations is reversed in the substrate- and product-bound forms, and that this reversal is one of the origins of the biochemical opposition. Structural polymorphs of the clock proteins, which have been confirmed by crystallography, NMR, and MD simulation, also play important roles in the substrate selectivity of CKIδ56 and the interaction between CRY1/2 and the CLOCK-BMAL1 complex57. In the cyanobacterial system, the rhythmic stacking/unstacking of C1- and C2-rings is coupled to the P-cycle of KaiC55. The MD simulation of KaiC suggests a large-scale conformational change during ADP release from the C2 domain58. These previous observations are mainly based on the MD simulations and experiments on nanosecond to microsecond time scales, which are much slower than the time scales targeted by the current QENS study.
The mechanism by which a single amino acid substitution can exert a notable effect on reaction- or even system-level temperature compensation deserves further investigation. There is growing experimental evidence that ordinary enzymes actively utilize internal motions, thereby increasing the efficiency of overall catalytic reactions59,60,61. Because functionally relevant fluctuations often refer to collective motions of atoms on slower time scales (μs to ms), care must be taken in discussing those motions in relation to the incoherent and fast (ps) dynamics detected in this study. However, several studies have demonstrated a linkage between ps–ns fluctuations and slower motions associated with catalytic reactions62,63,64. Thus, it is necessary to experimentally verify the possibility that the causality of temperature sensitivity in KaiC is still established on limited time scales longer than nanoseconds.
Methods
Expression and purification of Kai proteins
Glutathione S-transferase (GST)-tagged versions of Kai proteins were constructed in pGEX-6P-1. Each Kai GST-fusion protein was expressed in E. coli BL21(DE3) and purified as reported previously11,26.
ATPase assay
ATPase activities of KaiCWT and its mutants, dissolved in an H2O buffer (H1-buffer) including 20 mM Tris/HCl (pH 8.0), 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 1 mM EDTA, and 1 mM ATP, were measured at 303, 308, 313, and 318 K as previously reported11,15.
In vitro rhythm assay
P-cycles of KaiCWT and its mutants (0.2 mg/ml) were initiated by addition of KaiA (0.04 mg/ml) and KaiB (0.04 mg/ml) in H1-buffer at 303, 308, 313, and 318 K25. For KaiCF247A and KaiCQ361E, whose P-cycle were unstable or unobvious at the normal amount of KaiA, KaiA was added at a 2-fold higher concentration (0.08 mg/ml). Aliquots taken from the incubated samples were subjected to SDS-PAGE analysis. The relative abundances of four phosphorylation states of KaiC were quantified by densitometric image analysis of gel bands using the LOUPE software65.
Sample preparation for QENS
Every sample was prepared on site immediately before QENS measurements. KaiCWT and its mutants were purified on a gel-filtration column (Superdex 200 15/30, Cytiva) equilibrated with an H2O buffer (H2-buffer) containing 50 mM Tris/HCl (pH 8.0), 150 mM NaCl, 5 mM MgCl2, 3 mM DTT, 1 mM EDTA, and 20 mM ATP. Collected fractions were subjected to rapid buffer exchange in a D2O buffer (D2-buffer) containing 50 mM Tris/HCl (pD 7.6), 150 mM NaCl, 5 mM MgCl2, 3 mM DTT, 1 mM EDTA, and 20 mM ATP using a desalting column (HiPrep 50, Cytiva). KaiCWT, KaiCS258A, KaiCF247A, KaiCQ361E, KaiCN390A, KaiCF470Y, and KaiCDE were concentrated up to 15.0, 12.3, 14.6, 14.3, 16.9, 8.5, and 9.7 mg/mL, respectively. Each 1-mL sample was placed in a double-cylindrical aluminum cell with a sample thickness of 0.5 mm and sealed tightly with indium wire.
QENS experiments
QENS data were recorded using the near-backscattering spectrometer installed at beamline BL02 (DNA) in the Material and Life Science Experimental Facility of Japan Proton Accelerator Research Complex (MLF/J-PARC), Tokai, Ibaraki, Japan30. QENS spectra were recorded over an energy transfer range from −0.5 to 1.5 meV with energy resolution (12 μeV) enabling us to access motions faster than ~55 ps. QENS data were collected at 283, 293, 302, 310, 313, and 317 K with exposure times of 6–10 h for each condition (520−615 kW). The obtained S(Q,E) were corrected for detector efficiency using a vanadium standard, and intensities were normalized as relative intensities using the standard after subtracting the contributions of the empty cell. The background spectrum of the D2-buffer was subtracted from each sample spectrum on the basis of scaling factors calculated from neutron scattering cross-sections, as reported previously35,36. The temperature was controlled by an LS350 (Lakeshore) with He conductance gas through a GM refrigerator and heat transfer from a cartridge heater installed in a copper block at the top of a sample cell, while monitoring the temperature at the bottom of the cell.
Estimation of the Q10 values
Datasets with more than two temperature points were subjected to Arrhenius plot analysis, and the resulted activation energy (Ea) was converted to the Q10 value at 303 K using exp{(Ea/R)(10/T/(T + 10))}, where T and R represent temperature and the gas constant, respectively65. Other Q10 values were presented as the ratio of measurement results (R1 and R2) at two temperatures (T1 and T2) using Q10 = (R2/R1)10/(T2-T1).
Calculation of D global
The Dglobal values of KaiCWT were simulated at 302 and 313 K using a previously reported method39, the crystal structure (2GBL)32, and HYDROPRO66 (Supplementary Note 2).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Pittendrigh, C. S. Temporal organization: reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 55, 16–54 (1993).
Akiyama, S. Structural and dynamic aspects of protein clocks: how can they be so slow and stable? Cell. Mol. Life Sci. 69, 2147–2160 (2012).
Ode, K. L. & Ueda, H. R. Design principles of phosphorylation-dependent timekeeping in eukaryotic circadian clock. Csh. Perspect. Biol. 10, a028357 (2018).
Partch, C. L. Orchestration of circadian timing by macromolecular protein assemblies. J. Mol. Biol. 432, 3426–3448 (2020).
Narasimamurthy, R. & Virshup, D. M. The phosphorylation switch that regulates ticking of the circadian clock. Mol. Cell 81, 1133–1146 (2021).
Segel, I. H. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems (Wiley, 1975).
Blandamer, M. J. & Morris, S. H. Investigation into effect of temperature and added tert-butyl alcohol on dynamic properties of Belousov reaction. J. Chem. Soc. Farad. T. 1 71, 2319–2330 (1975).
Koros, E. Monomolecular treatment of chemical oscillation. Nature 251, 703–704 (1974).
Ruoff, P. Antagonistic balance in the oregonator—about the possibility of temperature-compensation in the Belousov-Zhabotinsky reaction. Phys. D. 84, 204–211 (1995).
Yoshikawa, K. Distinct activation-energies for temporal and spatial oscillations in the Belousov-Zhabotinskii reaction. B. Chem. Soc. Jpn 55, 2042–2045 (1982).
Mukaiyama, A., Ouyang, D. Y., Furuike, Y. & Akiyama, S. KaiC from a cyanobacterium Gloeocapsa sp. PCC 7428 retains functional and structural properties required as the core of circadian clock system. Int. J. Biol. Macromol. 131, 67–73 (2019).
Terauchi, K. et al. ATPase activity of KaiC determines the basic timing for circadian clock of cyanobacteria. Proc. Natl Acad. Sci. USA 104, 16377–16381 (2007).
Ito-Miwa, K., Furuike, Y., Akiyama, S. & Kondo, T. Tuning the circadian period of cyanobacteria up to 6.6 days by the single amino acid substitutions in KaiC. Proc. Natl Acad. Sci. USA 117, 20926–20931 (2020).
Tomita, J., Nakajima, M., Kondo, T. & Iwasaki, H. No transcription-translation feedback in circadian rhythm of KaiC phosphorylation. Science 307, 251–254 (2005).
Murayama, Y. et al. Tracking and visualizing the circadian ticking of the cyanobacterial clock protein KaiC in solution. EMBO J. 30, 68–78 (2011).
Isojima, Y. et al. CKI epsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc. Natl Acad. Sci. USA 106, 15744–15749 (2009).
Abe, J. et al. Atomic-scale origins of slowness in the cyanobacterial circadian clock. Science 349, 312–316 (2015).
Shinohara, Y. et al. Temperature-sensitive substrate and product binding underlie temperature-compensated phosphorylation in the clock. Mol. Cell 67, 783–798 (2017).
Ruoff, P., Rensing, L., Kommedal, R. & Mohsenzadeh, S. Modeling temperature compensation in chemical and biological oscillators. Chronobiol. Int. 14, 499–510 (1997).
Hatakeyama, T. S. & Kaneko, K. Generic temperature compensation of biological clocks by autonomous regulation of catalyst concentration. Proc. Natl Acad. Sci. USA 109, 8109–8114 (2012).
Kurosawa, G. & Iwasa, Y. Temperature compensation in circadian clock models. J. Theor. Biol. 233, 453–468 (2005).
Das, S., Terada, T. P. & Sasai, M. Single-molecular and ensemble-level oscillations of cyanobacterial circadian clock. Biophys. Physicobiol. 15, 136–150 (2018).
Sasai, M. Effects of stochastic single-molecule reactions on coherent ensemble oscillations in the KaiABC circadian clock. J. Phys. Chem. B 123, 702–713 (2019).
Ishiura, M. et al. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science 281, 1519–1523 (1998).
Nakajima, M. et al. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 308, 414–415 (2005).
Nishiwaki, T. et al. A sequential program of dual phosphorylation of KaiC as a basis for circadian rhythm in cyanobacteria. EMBO J. 26, 4029–4037 (2007).
Rust, M. J., Markson, J. S., Lane, W. S., Fisher, D. S. & O’Shea, E. K. Ordered phosphorylation governs oscillation of a three-protein circadian clock. Science 318, 809–812 (2007).
Bée, M. Quasielastic Neutron Scattering (Adam Hilger, 1988).
Grimaldo, M., Roosen-Runge, F., Zhang, F., Schreiber, F. & Seydel, T. Dynamics of proteins in solution. Q. Rev. Biophys. 52, 1–63.e7 (2019).
Shibata, K. et al. The performance of TOF near backscattering spectrometer DNA in MLF, J-PARC. JPS Conf. Proc. 2015 8, 036022 (2015).
Ouyang, D. Y. et al. Development and optimization of expression, purification, and ATPase assay of KaiC for medium-throughput screening of circadian clock mutants in cyanobacteria. Int. J. Mol. Sci. 20, 2789 (2019).
Pattanayek, R. et al. Analysis of KaiA-KaiC protein interactions in the cyano-bacterial circadian clock using hybrid structural methods. EMBO J. 25, 2017–2028 (2006).
Furuike, Y. et al. Elucidation of master allostery essential for circadian clock oscillation in cyanobacteria. bioRxiv Preprint at https://doi.org/10.1101/2021.08.30.457330 (2021).
Murayama, Y. et al. Low temperature nullifies the circadian clock in cyanobacteria through Hopf bifurcation. Proc. Natl Acad. Sci. USA 114, 5641–5646 (2017).
Fujiwara, S. et al. Ligation-dependent picosecond dynamics in human hemoglobin as revealed by quasielastic neutron scattering. J. Phys. Chem. B 121, 8069–8077 (2017).
Fujiwara, S. et al. Dynamical behavior of human alpha-synuclein studied by quasielastic neutron scattering. PLoS ONE 11, e0151447 (2016).
Gibrat, G., Assairi, F. L., Blouquit, Y., Craescu, C. T. & Bellissent-Funel, M. C. Biophysical study of thermal denaturation of apo-calmodulin: dynamics of native and unfolded states. Biophys. J. 95, 5247–5256 (2008).
Dee, D. R., Myers, B. & Yada, R. Y. Dynamics of thermodynamically stable, kinetically trapped, and inhibitor-bound states of pepsin. Biophys. J. 101, 1699–1709 (2011).
Pérez, J., Zanotti, J. M. & Durand, D. Evolution of the internal dynamics of two globular proteins from dry powder to solution. Biophys. J. 77, 454–469 (1999).
Stadler, A. M. et al. Picosecond dynamics in haemoglobin from different species: a quasielastic neutron scattering study. Biochim. Biophys. Acta 1840, 2989–2999 (2014).
Zaccai, G. et al. The fluctuating ribosome: thermal molecular dynamics characterized by neutron scattering. Sci. Rep. 6, 37138 (2016).
Gaspar, A. M., Appavou, M. S., Busch, S., Unruh, T. & Doster, W. Dynamics of well-folded and natively disordered proteins in solution: a time-of-flight neutron scattering study. Eur. Biophys. J. Biophys. 37, 573–582 (2008).
Grimaldo, M., Roosen-Runge, F., Zhang, F. J., Seydel, T. & Schreiber, F. Diffusion and dynamics of gamma-globulin in crowded aqueous solutions. J. Phys. Chem. B 118, 7203–7209 (2014).
Matsuo, T., Tominaga, T., Kono, F., Shibata, K. & Fujiwara, S. Modulation of the picosecond dynamics of troponin by the cardiomyopathy-causing mutation K247R of troponin T observed by quasielastic neutron scattering. BBA-Proteins Proteom. 1865, 1781–1789 (2017).
Caronna, C., Natali, F. & Cupane, A. Incoherent elastic and quasi-elastic neutron scattering investigation of hemoglobin dynamics. Biophys. Chem. 116, 219–225 (2005).
Shrestha, U. R. et al. Effects of pressure on the dynamics of an oligomeric protein from deep-sea hyperthermophile. Proc. Natl Acad. Sci. USA 112, 13886–13891 (2015).
Marion, J. et al. Pressure-induced molten globule state of human acetylcholinesterase: structural and dynamical changes monitored by neutron scattering. Phys. Chem. Chem. Phys. 17, 3157–3163 (2015).
Golub, M. et al. High hydrostatic pressure specifically affects molecular dynamics and shape of low-density lipoprotein particles. Sci. Rep. 7, 46034 (2017).
Fujiwara, S., Matsuo, T., Sugimoto, Y. & Shibata, K. Segmental motions of proteins under non-native states evaluated using quasielastic neutron scattering. J. Phys. Chem. Lett. 10, 7505–7509 (2019).
Kataoka, M. et al. Dynamical and structural modifications of staphylococcal nuclease on C-terminal truncation. Phys. B 266, 20–26 (1999).
Kataoka, M. et al. Low energy dynamics of globular proteins studied by inelastic neutron scattering. J. Phys. Chem. Solids 60, 1285–1289 (1999).
Bu, Z. M. et al. A view of dynamics changes in the molten globule-native folding step by quasielastic neutron scattering. J. Mol. Biol. 301, 525–536 (2000).
Grimaldo, M. et al. Hierarchical molecular dynamics of bovine serum albumin in concentrated aqueous solution below and above thermal denaturation. Phys. Chem. Chem. Phys. 17, 4645–4655 (2015).
Fujiwara, S., Plazanet, M., Matsumoto, F. & Oda, T. Internal motions of actin characterized by quasielastic neutron scattering. Eur. Biophys. J. Biophys. 40, 661–671 (2011).
Chang, Y. G., Tseng, R., Kuo, N. W. & LiWang, A. Rhythmic ring-ring stacking drives the circadian oscillator clockwise. Proc. Natl Acad. Sci. USA 109, 16847–16851 (2012).
Philpott, J. M. et al. Casein kinase 1 dynamics underlie substrate selectivity and the PER2 circadian phosphoswitch. eLife 9, e52343 (2020).
Fribourgh, J. L. et al. Dynamics at the serine loop underlie differential affinity of cryptochromes for CLOCK:BMAL1 to control circadian timing. eLife 9, e55275 (2020).
Hong, L., Vani, B. P., Thiede, E. H., Rust, M. J. & Dinner, A. R. Molecular dynamics simulations of nucleotide release from the circadian clock protein KaiC reveal atomic-resolution functional insights. Proc. Natl Acad. Sci. USA 115, E11475–E11484 (2018).
Eisenmesser, E. Z. et al. Intrinsic dynamics of an enzyme underlies catalysis. Nature 438, 117–121 (2005).
Terazima, M. Enhanced conformational fluctuations during protein reactions. Chem. Lett. 48, 802–810 (2019).
Agarwal, P. K. Role of protein dynamics in reaction rate enhancement by enzymes. J. Am. Chem. Soc. 127, 15248–15256 (2005).
Henzler-Wildman, K. A. et al. A hierarchy of timescales in protein dynamics is linked to enzyme catalysis. Nature 450, 913–916 (2007).
Hawkins, R. J. & McLeish, T. C. B. Coupling of global and local vibrational modes in dynamic allostery of proteins. Biophys. J. 91, 2055–2062 (2006).
Agarwal, P. K. A biophysical perspective on enzyme catalysis. Biochemistry 58, 438–449 (2019).
Furuike, Y., Abe, J., Mukaiyama, A. & Akiyama, S. Accelerating in vitro studies on circadian clock systems using an automated sampling device. Biophys. Physicobiol. 13, 235–241 (2016).
Ortega, A., Amoros, D. & de la Torre, J. G. Prediction of hydrodynamic and other solution properties of rigid proteins from atomic- and residue-level models. Biophys. J. 101, 892–898 (2011).
Acknowledgements
We thank Dr. K. Shibata for his kind support for the trial QENS experiment, and Dr. M. Kataoka and Dr. H. Kamikubo for their discussions and critical comments on the manuscript. This study was partly supported by Grants-in-Aid for Scientific Research (17H06165 to S.A.). The QENS experiments using BL02 (DNA) at the Materials and Life Science Experimental Facility of the J-PARC were performed under user programs (Proposal No. 2017B0123, 2018B0244, 2019A0308, and 2020B0073).
Author information
Authors and Affiliations
Contributions
Y.F. and S.A. designed the study; Y.F., D.O., T.M., and A.M. prepared samples; Y.F. and D.O. conducted biochemical assays; Y.F., T.T., T.M., Y.K., S.F., and S.A. performed QENS experiments; Y.F., T.T., T.M., Y.K., S.F., and S.A. analyzed QENS data; and S.A., S.F., and Y.F. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Physics thanks Andreas Stadler, Yong-Ick Kim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Furuike, Y., Ouyang, D., Tominaga, T. et al. Cross-scale analysis of temperature compensation in the cyanobacterial circadian clock system. Commun Phys 5, 75 (2022). https://doi.org/10.1038/s42005-022-00852-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42005-022-00852-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.