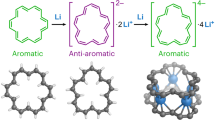
Abstract
Aromaticity can be assigned by Hückel’s rule, which predicts that planar rings with delocalized (4n + 2) π-electrons are aromatic, whereas those with 4n π-electrons are antiaromatic. However, for neutral rings, the maximal value of “n” to which Hückel’s rule applies remains unknown. Large macrocycles exhibiting global ring current can serve as models for addressing this question, but the global ring current are often overshadowed in these molecules by the local ring current of the constituent units. Here, we present a series of furan-acetylene macrocycles, ranging from the pentamer to octamer, whose neutral states display alternating contributions from global aromatic and antiaromatic ring currents. We find that the odd-membered macrocycles display global aromatic characteristics, whereas the even-membered macrocycles display contributions from globally antiaromatic ring current. These factors are expressed electronically (oxidation potentials), optically (emission spectra), and magnetically (chemical shifts), and DFT calculations predict global ring current alternations up to 54 π-electrons.
Similar content being viewed by others
Introduction
Planar cyclic molecules with π-conjugation display aromaticity or antiaromaticity depending on their number of π-electrons, 4n + 2 or 4n respectively, according to Hückel’s rule1. While aromaticity is expressed in energetic, geometric, and optical terms2,3, it is most commonly quantified in magnetic terms, with either diatropic or paratropic ring currents for aromatic or antiaromatic compounds, respectively4,5. It is estimated that, for neutral annulenes, Hückel’s rule is effective for ≤30 π-electrons, but theoretically it can also be valid for larger annulenes6,7. However, distortion from planarity with increased degrees of freedom, as well as increasing numbers of stereoisomers, prevents the experimental validation of this assumption. π-Conjugated macrocycles, which are composed of smaller aromatic rings in a cyclic arrangement, are shape-persistent, often more stable than annulenes, and are expected to show similar trends and thus produce aromatic or antiaromatic global ring currents8,9,10,11.
Methine-spaced macrocycles, such as porphyrins, have a distinct quinoid character, which is expressed in a double bond between the methine and the α-carbon12. Consequently, they show aromatic or antiaromatic global ring currents, depending on their size13,14. For example, Wu’s group demonstrated that for [m]-cycloparaphenyl-methines ([m]CPPM, where m is the number of linked rings, in this case, phenyl groups, comprising the macrocycle; Fig. 1), the hexamer [6]CPPM displays global antiaromaticity at low temperatures, whereas [8]CPPM is distorted from planarity and ‘escapes’ antiaromaticity15,16. Anand’s group demonstrated different absorption spectra for furan-containing porphyrinoid macrocycles containing 30 π- and 40 π-electrons17. Anderson’s group demonstrated that acetylene-spaced porphyrin nanorings display global aromaticity in different oxidation states, even for 162 π-electrons18, including in their excited state19. However, these global ring currents were not detected in their neutral ground state20.
In contrast, neutral non-quinoid macrocycles, connected by ethylene, acetylene, or any other linker with an even number of carbon atoms, are not commonly reported to show global ring current21. For example, [m]CPPA series members have 6 π-electrons per repeat unit that can contribute to the global ring current. Therefore, global current in even-membered [m]CPPA macrocycles consist of 4n π-electrons, whereas odd-membered [m]CPPA macrocycles have 4n + 2 π-electrons. As a result, the series members should alternate between global aromatic and antiaromatic ring currents. However, the 1H-NMR chemical shift does not display the expected alternating trend for odd and even m22. One explanation is that the local aromaticity of the benzene ring overshadows any global effects. Therefore, reducing local aromaticity by replacing the benzene rings with less-aromatic thiophene or furan units is expected to increase the expression of global ring current. However, thiophene-acetylene macrocycles ([m]-TA, Fig. 1) have been reported only for even-membered macrocycles, and therefore the expected aromatic/antiaromatic alternation of global ring current cannot be explored experimentally23.
We have previously introduced oligofurans, which display weaker aromaticity and therefore stronger quinoid character compared with their thiophene analogs24,25. The lower aromaticity of furan is expressed in greater chemical reactivity (for example, as a diene in Diels-Alder cycloaddition)26,27, shorter interring distances24, as well as in magnetic ring currents. The exo angle of furan is predicted to favor macrocycles with significantly smaller sizes than can be obtained from thiophenes28. Macrocyclic furans ([m]F, Fig. 1) of smaller sizes are planar and display a contribution from globally antiaromatic ring current in their neutral state and from globally aromatic ring current in their dicationic state29,30,31. Larger macrocyclic furans are distorted out of planarity, thereby avoiding global antiaromaticity32. However, since each furan ring contributes 4 π-electrons to the global ring current of macrocyclic furans, they are expected to be globally antiaromatic regardless of the number of rings. Märkl et al., explored oxaporphyrin with 4n and 4n + 2 π-electrons, however the presence of multiple isomers prevented drawing clear conclusions regarding the contribution of global aromatic ring currents33,34. Therefore, the size-dependency of Hückel’s rule in neutral macrocycles remains an open question.
Here, we introduce a series of furan-acetylene macrocycles, Cm with m = 5–8 furan rings (Fig. 1). Unlike previous macrocyclic furans with 4n π-electron systems, the 6 π-electrons per repeat unit in the Cm series is expected to display alternating global aromatic and antiaromatic ring currents, for odd and even m, respectively. Indeed, our experimental results show that Cm series members in their neutral state exhibit alternating properties for odd- and even-membered rings. All the magnetic (NMR), photophysical (absorption and fluorescence spectra), and electrochemical properties of these furan-acetylenes indicate a contribution from global antiaromaticity in even-membered macrocycles, whereas odd-membered macrocycles are globally aromatic. Nucleus independent chemical shifts (NICS) calculations support this trend, predicting that the contribution of the global ring current is significant up to 54 π-electrons in neutral Cm. Overall, this work highlights the prospect of furan serving as a probe for global aromatic effects in macrocycles.
Results and discussion
Synthesis and structure
To synthesize the Cm series, comprising both odd- and even-membered macrocycles, we adopted the monomer rather than the oligomer approach. For [m]TA (Fig. 1), the monomer approach is unlikely to produce small macrocycles, since the angle between the α substituents is 150°, which is ideal for 12 membered macrocycles. In contrast, the angle between the α substituents of furan is 125°, which should be ideal for 6–7 membered macrocycles28. Therefore, to obtain a sequential series of small macrocycles with 6 π-electrons per unit, macrocyclization of 2-ethynyl-furan units was the method of choice.
The synthesis of Cm is depicted in Fig. 2. 2-Bromo-3-hexylfuran (1)35, was coupled to triisopropylsilyl-acetylene by Sonogashira coupling using triethylamine as a solvent in the presence of bis(triphenylphosphine)palladium(II) dichloride or copper iodide to obtain 2 with a 78% yield. The hexyl-groups were found to be important for the solubility of both the intermediates during the macrocyclization step and the final macrocycles. Lithiation of 2 with lithium-diisopropylamine followed by dropwise addition of a solution of I2 in tetrahydrofuran afforded 3, and the triisopropylsilyl group was subsequently removed with tetra-n-butylammonium fluoride to afford 4 with an overall yield of 60%.
Reagents and conditions: (a) TIPS-acetylene, (Pd(PPh3)2Cl2), Et3N, reflux, 2 days; (b) I2 (3 eq), lithium diisopropylamine (1 M), THF, -78 °C; (c) tetrabutylammonium fluoride (1 M), THF, 3 h; (d) Pd(PPh3)4 (10% mol), CuI (3% mol), toluene:diisopropylethylamine (1:1), 60 °C, 4 days. Et ethyl; Ph phenyl; TIPS triisopropylsilyl; THF tetrahydrofuran.
For the macrocyclization of 4 using Sonogashira coupling, we attempted modification of several parameters, including solvent, base, temperature, and reactant concentration (see Supplementary Table 1). We found that heating a toluene/diisopropylethylamine solution of 4 (22 mM) to 60 °C with palladium-tetrakis(triphenylphosphine) (10% mol) and copper (I) iodide (3% mol) for four days produced C5–C8 with an overall yield of 35%. The macrocycles were then separated using size exclusion chromatography. The size distribution is in-line with the calculated strain (Fig. 3), where C7 displays the lowest strain energy (3.1 kcal mol−1) and highest yield (13%) and C5 displays the highest strain energy (10.1 kcal mol−1) and lowest yield (<1%).
Crystals of C6 and C7 were grown by slow evaporation from hexane and chloroform solutions. The solid-state structures are displayed in Fig. 4. The average dihedral angle (see Supplementary X-Ray Crystallography S6.1) for C6 is 26°, and in the solid state it adopts a chair conformation, similar to cyclohexane. C7 is significantly more planar, with an average dihedral angle of 12°, which also corresponds to the abovementioned lower strain energy. The calculated structures for Cm are nearly planar for all sizes, with an average dihedral angle of 1° or less. We note that, as the barrier to rotation around the triple bond is low, the average conformation in solution is also expected to be planar or nearly-planar, as indicated by the calculated structures. The C ≡ C bond lengths do not display any significant changes (computationally or experimentally) between the different members of the Cm series, and this also applies to other bond distances in the π-conjugated backbone. This is consistent with previously reported examples, in which alternating global aromaticity in large (>20 π-electrons) π-conjugated macrocycles was not expressed in the bond distances20.
Redox properties
Figure 5 presents the cyclic voltammograms of Cm in dichloromethane. The oxidation potential (anodic peak, Epa) of C5 is 0.25 V higher compared with C6 (Epa = 0.77 V and 0.52 V for C5 and C6, respectively). C6 displays two distinct reversible oxidation peaks. C7 also exhibits a higher oxidation state compared with C6, although the difference is only 0.07 V (Epa = 0.59 V) and the oxidation potential for C8 is again lower than C7 (Epa = 0.52 V). C6 stands out as the only member of the Cm series that exhibits two distinct reversible oxidation peaks, and we have previously observed similar behavior from the antiaromatic 8-membered furan macrocycle30. Therefore, C6 is the only member that exhibits a significant contribution from global antiaromatic ring current. The antiaromatic character of C6 is quite pronounced, whereas the cyclic voltammogram of C8 is not affected significantly by the global antiaromaticity.
As a result of their global aromatic character, odd-membered Cm are expected to exhibit higher oxidation potentials compared with their even-membered analogs, since oxidation to the cation is expected to result in the loss of aromaticity, and further oxidation to the dication is expected to result in the emergence of an antiaromatic contribution. Thus, the alternating trend of the oxidation potentials is consistent with the expected trend of global aromaticity/antiaromaticity. We note that the redox potential of charged macrocycles was previously suggested as a tool to probe global antiaromaticity36.
The calculated (M06/6-311 G(d)) energy of the highest occupied molecular orbital (HOMO) of C5 is c.a. 0.3 eV lower than that of C6 (−5.41 eV vs. −5.12 eV, respectively), which corresponds to the abovementioned trend in the experimentally observed oxidation potentials. The HOMO of C7 (−5.27 eV) is even lower compared with C6 and the HOMO of C8 (−5.11 eV) is higher compared with C7. Overall, the alternating behavior of the HOMO energy levels with size matches the trends observed experimentally in the electrochemical oxidation. The difference between the oxidation potentials of C6 and C8 can be explained by the difference between their calculated HOMOs, which is 0.3 eV higher for C8. It is interesting to note that macrocycles with formal antiaromatic systems were previously applied as active materials in batteries since they display stable redox states37.
To study further the stability of the oxidized species, we attempted to chemically oxidize Cm with an excess of nitrosonium hexafluoroantimonate (NOSbF6), which was found to be an effective oxidant of macrocyclic furans28. Oxidation of C6 resulted in two sets of spectra, depending on the amount of oxidant added. The first set of spectra, showing a broad absorption band at 1744 nm, and can be attributed to the cation radical, whereas the second set with bands at 1130 nm and 550 nm, can be attributed to the dication (see Supplementary Fig. 46). For C8, stepwise oxidation resulted in only one specie, which can be attributed to the cation radical. In contrast, attempts to oxidize C5 and C7 resulted in quick decomposition, even under an inert atmosphere (N2-filled glovebox), with no apparent absorption bands that can be attributed to cationic or dicationic species. The greater stabilization upon oxidization of C6 and C8 compared with C5 and C7 is further evidence of the aromatic character of dicationic C6 and cationic C8.
Photophysical properties
The absorption and emission spectra of Cm are depicted in Fig. 6. C5 and C6 display relatively sharp absorption bands, with a weak S0→S1 transition. For C5, the vibronic shoulders for the S0→S1 transition can be observed at 415 nm and 431 nm. We note that, although C5 is more rigid than C6, the relative intensity of the S0→S1 transition is stronger (by 15% for C5 and 8% for C6) compared with the S0→Sn transition (n > 1). This indicates the involvement of additional factors in the oscillator strength. C7 and C8 display broader absorption spectra, corresponding to their more flexible structures. In chloroform, the absorption spectra of C6 and C7 exhibit concentration dependence above 10−5 M (see Supplementary Figs. 39a, 41). We, therefore, measured the absorption and emission spectra at lower concentrations as well as in toluene, where no concentration dependence was observed (see Supplementary Figs. 38–40, 42b).
Normalized absorption (blue) and emission (red) spectra of the Cm series in chloroform. Right: Emission by chloroform solutions of Cm irradiated at 365 nm. The gray trace follows the experimental emission maxima. The green trace represents the S1 → S0 transition calculated at the TD-DFT/CAM-B3LYP/6-311 G(d) level of theory, showing the same alternating trend.
The emission spectra for C5 and C6 differ markedly from each other: whereas the emission maxima (λem) for C5 is 490 nm, there is a significant bathochromic shift of 72 nm (0.32 eV) for C6. This also manifests in a clear difference in color upon irradiation in black light (blue for C5 and orange for C6, Fig. 6). C7 again displays a hypochromic shift compared with C6, with emission maxima of 513 nm, and the emission maxima for C8 is again slightly bathochromic (λem = 533 nm). We note that, although the differences are large, this should be taken with caution, as increasing macrocycle size also results in increased flexibility, which in turn affects the emission properties. The fluorescence quantum efficiencies are small (5–6%) for the rigid C5 and C6, and increase significantly for C7 and C8 (35 and 37%, respectively). In addition, the emission maxima seem to occur in different vibronic states. Nevertheless, time-dependent density functional theory (TD-DFT) calculations display the same alternating trend as observed experimentally, with S1→S0 transitions at longer wavelengths for even-membered macrocycles (478 nm for C5 and 544 nm for C6, calculated at the CAM-B3LYP/6-31 G(d) level). The green trace in Fig. 6 represents the calculated emission maxima for Cm, showing the same alternating trend as the experimental emission maxima (gray trace). Overall, the emission spectra show alternating behavior, with even-membered rings bathochromically-shifted compared with the odd-membered rings.
We utilized DFT to calculate the behavior of the Cm series to understand better the alternating behavior of its members. The frontier molecular orbitals for Cm are depicted in Fig. 7. The gap between the highest occupied and lowest unoccupied molecular orbitals (the HOMO–LUMO gap) is smaller for compounds with a globally antiaromatic character compared with their aromatic counterparts, which is consistent with the alternating fluorescence maxima observed above. This was previously rationalized by Rabinovitz’s group for polyaromatic compounds, who reasoned that antiaromaticity is an antibonding property, and therefore the LUMO is stabilized, whereas the HOMO is destabilized38.
As can be observed in Fig. 7, the odd-membered macrocycles (C5 and C7) display degenerate HOMO and LUMO levels, and even-membered macrocycles (C6 and C8) displayed degeneracy of the HOMO-1 and LUMO + 1 levels. This is consistent with the trend for expanded porphyrins previously observed by Sessler and Kim39, who suggested optical properties as a parameter for aromaticity, and reported that the difference between aromaticity and antiaromaticity is expressed in red-shifted and weaker emission in globally antiaromatic macrocycles compared with globally aromatic macrocycles.
The difference in degeneracy is explained by the localized π-electron character of the structures with antiaromatic contribution compared with the delocalized electron structure of aromatic macrocycles (it was previously reported that, in antiaromatic systems, π-overlap favors localization of the double bonds)40. The HOMO–LUMO gap also alternates between odd and even Cm, with the odd-membered (aromatic) C5 and C7 displaying larger gaps (3.42 and 3.11 eV, respectively) compared with the even-membered (antiaromatic) C6 and C8 (2.84 and 2.77 eV, respectively). We note that for even Cm, both the HOMO and LUMO levels have the same number of nodal planes, as expected for antiaromatic compounds on the basis of basic symmetry arguments41.
Magnetic ring current
The 1H-NMR spectra of Cm show the dependency of the chemical shifts on macrocycle size. In particular, the protons located at the β-position of the furan rings experience an upfield shift of 0.28 ppm for C6 (6.41 ppm) compared with C5 (6.69 ppm, Fig. 8 solid line). C7 shows a downfield shift of 0.16 ppm compared with C6, and the chemical shift of C8 is nearly identical to that of C7. The same trend is also observed for the methylene protons of the hexyl chain attached to the furan ring, and to nearly the same extent (see Supplementary Fig. 52). For example, the chemical shift for the methylene protons of C5 is 2.65 ppm and of C6 is 2.44 ppm. By comparison, in the acyclic compound 2 (Fig. 2), the chemical shift of the methylene protons is 2.49 ppm, which is almost identical to the value for C8 (2.50 ppm). The calculated chemical shifts at the M06-2X/6-311 G(d) level show the same trend, with the even-membered Cm members experiencing an upfield shift (Fig. 8, dashed line). This can be rationalized by the diatropic and paratropic global ring currents affecting the external protons in global aromatic and antiaromatic currents, respectively.
To gain a better understanding of the underlying experimentally-observed trends, we evaluated the extent of the global effects by means of NICS calculations. NICS(1)zz probes the ring current 1 Å above the center of the ring of interest; negative values indicate a diatropic ring current (an aromatic system), whereas positive values suggest a paratropic ring current (an antiaromatic system)42. We have chosen to monitor the NICS(1)zz values for the furan rings, since the comparison of ring current for different ring sizes will necessarily result in different NICS values, as the distance from the dummy atom varies. First, the NICS(1)zz values for each member of the Cm series were calculated. Figure 9 displays a map of the zz components 1 Å above the average molecular plane. Although the effects are subtle in the neutral form, the differences between C5, C6, and C7 are clearly observed in the interior area of the macrocycles: C5 and C7 show very weak aromatic (negative) values, whereas the larger red area in C6 indicates a contribution from global antiaromatic current. The same trend also applies to C8, although in a lesser extent. We note that the aromatic furan units should induce an external deshielding, which can explain why the internal space of the odd-membered macrocycles is not aromatic (see Supplementary Figs. 61–64)41. In contrast to the NICS(1)zz calculations, plots of the anisotropy of the current (induced) density (ACID) only show global ring currents for the macrocycles in their dicationic states (Cm2+). Unlike Cm, the NICS(1)zz maps for [m]CPPA do not display any visible global aromaticity or antiaromaticity regardless of their size (see Supplementary Fig. 65).
Size dependency of Hückel’s rule
Although the 4n + 2 rule applies to small annulenes, the critical value of n is still debatable. For example, the aromatic stabilization energy of neutral annulenes is effective up to n = 6–7 (26–30 π-electrons)6. However, although magnetic criteria are perhaps the commonly applied tool for the quantification of aromaticity, the attenuation of chemical shifts with size was not reported for neutral macrocycles. In the current work, we show that, although the properties are clearly attenuated, alternation in magnetic and electronic properties can be observed experimentally in the Cm series up to 48 π-electrons (m = 8). To gain insight into the rate of attenuation for sizes larger than m = 8, the NICS(1) values of Cm members were calculated for m = 5–12. For comparison, we also calculated the NICS(1) for the same sized [m]CPPA series members (Fig. 10, red trace). Given that each unit of a member of the [m]CPPA series has 6 π-electrons, [m]CPPA is expected to display alternating behavior similar to that of Cm. We note that attenuation with size is dependent on the functional used. It is known that π-conjugated systems suffer from over-delocalization when B3LYP is used. Following a recent discussion43,44, we investigated several functionals and compared the values with the experimental chemical shift. We found that M06-2X provided the best agreement with the experimental values, and therefore applied this functional in all NICS calculations (see Supplementary Table 5 and Supplementary Fig. 66 for NICS data calculated at different computational levels).
The average NICS(1) values for the furan rings are more negative for odd-members C5 and C7, compared with C6 and C8, indicating a stronger local aromatic character for the former. The observed alternating trend provides additional evidence of the global ring currents, even for macrocycles in the neutral form. The alternating trend decays, but still clearly persists up to m = 9 (54 π-electrons). This is a significantly larger value than was observed previously for neutral macrocycles in the S0 state. By contrast, alternation in the NICS(1) values of [m]CPPA attenuates rapidly with size, and no effective change can be observed beyond m = 7. The difference between the two systems can be attributed to the lower local aromaticity of furan amplifying the global properties. It is interesting to analyze these results in the context of ‘concealed aromaticity’ as recently coined by Glöcklhofer45. The global properties of Cm are revealed predominantly upon oxidation (by their oxidation potentials and stability) and in the excited state (by fluorescence maxima), and can therefore be categorized as both type-I and type-II concealed aromaticity. However, in contrast to the [m]CPPA series, the aromatic character of Cm is also revealed in the neutral state (by chemical shift), highlighting the importance of lowering local aromaticity to reveal global properties.
Conclusions
In summary, we have introduced a series of π-conjugated furan-acetylene macrocycles in which the low aromaticity of furan manifests as distinct global effects in the neutral state. The odd-membered macrocycles, consisting of 4n + 2 π-electrons, show a global aromatic character whereas the even membered macrocycles (4n π-electrons) show a global antiaromatic character. These effects are expressed electronically (alternating oxidation potentials), optically (alternating emission maxima) and magnetically (alternating chemical shifts). NICS(1) calculations support the experimental observations. The differences are more pronounced for the smaller macrocycles, C5 and C6, and are attenuated, yet still observable, for C7 and C8. We show that the size-dependency of Hückel’s rule derives from the local aromaticity of the constituent unit. For macrocyclic furan acetylenes, this dependency can theoretically proceed up to 54 π-electrons, whereas for [m]CPPA, the alternation decays rapidly, and the effective size is 42 π-electrons. Overall, this work shows the first example of global aromaticity switching in neutral non-quinoidal macrocycles, and highlights the potential of macrocyclic furans to serve as shape persistent models for large annulenes.
Methods
General information
All reagents and chemicals were obtained from commercial suppliers and used as received without further purification. Flash chromatography was performed using CombiFlash SiO2 columns. 1H and 13C NMR spectra were recorded in solution on Brucker-AVIII 400 MHz and 500 MHz spectrometers using tetramethylsilane as the external standard. The spectra were recorded using chloroform-d as the solvent. Chemical shifts are expressed in δ units. High resolution mass spectra were measured on a high resolution quadrupole time-of-flight liquid chromatograph mass spectrometer (MS) and Waters Micromass GCT_Premier MS using electrospray ionization. Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) spectra were acquired using an MALDI-TOF/TOF autoflex speed MS (Bruker Daltonilk GmbH, Bremen, Germany), which is equipped with a smartbeam-II solid-state laser (modified Nd:YAG laser; λ = 355 nm). The instrument was operated in positive ion, reflectron mode. The accelerating voltage was 21.0 kV. The delay time was 130 ns. Laser fluence were optimized for each sample. The laser was fired at a frequency of 2 kHz and spectra were accumulated in multiples of 500 laser shots, with 1500 shots in total. 2-[(2E)-3-(4-tert-Butylphenyl)-2-methylprop2-enylidene] malononitrile (DCTB) matrix solutions were prepared to a concentration of 20 mg mL−1 in dichloromethane (DCM). Sample solutions were prepared to an approximate concentration of 5 mg mL−1 in DCM. Sample and matrix solutions were premixed at a ratio of 1:9 or 1:40 (v/v). A volume of 1 μL of this mixture was disposed on a MALDI steel target plate. After evaporation of the solvent, the target was inserted into the mass spectrometer. Preparative size exclusion chromatography was performed in chloroform solution at room temperature using a Recycling Preparative HPLC LaboACE LC-7080 through JAIGEL-2HR and JAIGEL-2.5HR columns connected in series. 2-Bromo-3-hexylfuran (1) was synthesized according to literature procedures35. Absorption spectra were recorded on an Agilent Technologies Cary 5000 UV-Vis-NIR spectrophotometer. Fluorescence and excitation spectra were performed on Horiba Scientific Fluoromax-4 spectrofluorometer. All spectroscopic experiments were performed using standard quartz cuvettes of path length 1 cm and solutions prepared in spectroscopic grade solvents. The excitation laser used was 330 nm with a pulse width of less than 1.4 ns. The synthetic pathway for 4 is described in the Supplementary Synthesis section (Supplementary Fig. 1). NMR and MS spectra are provided for all compounds: see Supplementary Figs. 2–37. Absorption and emission spectra for C5-C8 are provided in Supplementary Figs. 38–46. Circular voltammograms are provided in Supplementary Figs. 47–50. Calculated chemical shifts, ACID, NICS, and VIST plots for C5-C8 are provided in Supplementary Figs. 51–71. Optimization conditions for macrocyclization are provided in Supplementary Table 1. Photophysical properties for C5-C8 are provided in Supplementary Table 2. Calculated formation energies and MO energies are provided in Supplementary Tables 3–4. Calculated NICS values, strain energies and emission spectra are provided in Supplementary Tables 5–7, respectively. Crystallographic data for C6 and C7 is provided in Supplementary Table 8.
Synthetic procedure for C m
2-Ethynyl-3-hexyl-5-iodo-furan (4) (1 g, 3.31 mmol), tetrakis(triphenylphosphine)palladium (390 mg, 10%mol) and CuI (18 mg, 3%mol) were dissolved in a mixture of 75 mL of dry toluene and 75 mL of distilled N,N-diisopropylethylamine under an inert atmosphere, heated to 60 °C, and the reaction mixture was stirred for 3 days. Afterward, the reaction mixture was allowed to reach room temperature and was subsequently filtered over celite. The filtrate was evaporated and the residue obtained was purified by flash column chromatography on silica gel using a mixture of DCM and hexane (9:1) as eluent to give a mixture of macrocycles. The mixture was separated using gel permutation chromatography and chloroform as the eluent to give C5 (1.38 mg, <1%), C6 (10.4 mg, 10.5%), C7 (10.9 mg, 13%), and C8 (8.5 mg, 11.5%).
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Center, under deposition numbers CCDC 2191813 (C6) and 2191812 (C7). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Synthetic and characterization data for all reported compounds as well as computational details are included in the Supplementary Information file under “Supplementary Methods”. Cartesian coordinates for calculated structures are included in Supplementary Data 1. The crystallographic information file (CIF) for C6 and C7 are included in Supplementary Data 2 and 3, respectively.
References
Hückel, E. Quantentheoretische Beiträge zum Benzolproblem. Z. Physik 70, 204–286 (1931).
Gleiter, R. & Haberhauer, G. Aromaticity and other conjugation effects. (Wiley-VCH, 2012).
Krygowski, T. M., Cyrañski, M. K., Czarnocki, Z., Häfelinger, G. & Katritzky, A. R. Aromaticity: a theoretical concept of immense practical importance. Tetrahedron 56, 1783–1796 (2000).
Chen, Z., Wannere, C. S., Corminboeuf, C., Puchta, R. & Schleyer, PvonR. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 105, 3842–3888 (2005).
von Schleyer, P. R. & Jiao, H. What is aromaticity? Pure and Applied Chemistry 68, 209–218 (1996).
Wannere, C. S. & Schleyer, PvonR. How aromatic are large (4n + 2)π annulenes? Org. Lett. 5, 865–868 (2003).
Andes Hess, B., Schaad, L. J. & Holyoke, C. W. On the aromaticity of annulenones. Tetrahedron 28, 5299–5305 (1972).
Iyoda, M., Yamakawa, J. & Rahman, M. J. Conjugated macrocycles: concepts and applications. Angew. Chem. Int. Ed. 50, 10522–10553 (2011).
Wössner, J. S. et al. [n]Cyclodibenzopentalenes as antiaromatic curved nanocarbons with high strain and strong fullerene binding. J. Am. Chem. Soc. 143, 12244–12252 (2021).
Wang, J., Zhang, X., Jia, H., Wang, S. & Du, P. Large π-extended and curved carbon nanorings as carbon nanotube segments. Acc. Chem. Res. 54, 4178–4190 (2021).
Sprachmann, J. et al. Antiaromatic covalent organic frameworks based on dibenzopentalenes. J. Am. Chem. Soc.145, 2840–2851 (2023).
Sessler, J. L. & Seidel, D. Synthetic expanded porphyrin chemistry. Angew. Chem. Int. Ed. 42, 5134–5175 (2003).
Ito, T. et al. Gram‐scale synthesis of nickel(II) norcorrole: the smallest antiaromatic porphyrinoid. Angew. Chem. Int. Ed 51, 8542–8545 (2012).
Saito, S. & Osuka, A. Expanded porphyrins: intriguing structures, electronic properties, and reactivities. Angewandte Chemie International Edition 50, 4342–4373 (2011).
Li, Z. et al. [6]Cyclo- para -phenylmethine: an analog of benzene showing global aromaticity and open-shell diradical character. J. Am. Chem. Soc. 141, 16266–16270 (2019).
Li, Z. et al. [8]Cyclo-para-phenylmethine as A Super-Cyclooctatetraene: Dynamic Behavior, Global Aromaticity, and Open-Shell Diradical Character in The Neutral and Dicationic States. Angew. Chem. Int. Ed. 61, e202210697 (2022).
Reddy, J. S., Mandal, S. & Anand, V. G. Cyclic oligofurans: one-pot synthesis of 30π and 40π expanded porphyrinoids. Org. Lett. 8, 5541–5543 (2006).
Rickhaus, M. et al. Global aromaticity at the nanoscale. Nat. Chem. 12, 236–241 (2020).
Peeks, M. D. et al. Aromaticity and antiaromaticity in the excited states of porphyrin nanorings. J. Phys. Chem. Lett 10, 2017–2022 (2019).
Jirásek, M., Anderson, H. L. & Peeks, M. D. From macrocycles to quantum rings: does aromaticity have a size limit? Acc. Chem. Res. 54, 3241–3251 (2021).
Rimmele, M. et al. Functional group introduction and aromatic unit variation in a set of π-conjugated macrocycles: revealing the central role of local and global aromaticity. Org. Chem. Front. 8, 4730–4745 (2021).
Kawase, T. et al. Cyclic [5]Paraphenyleneacetylene: synthesis, properties, and formation of a ring-in-ring complex showing a considerably large association constant and entropy effect. Angew. Chem. Int. Ed 46, 1086–1088 (2007).
Shimizu, H. et al. Synthesis, structures, and photophysical properties of π-expanded oligothiophene 8-mers and their saturn-like C60 complexes. J. Am. Chem. Soc. 137, 3877–3885 (2015).
Gidron, O. & Bendikov, M. α-Oligofurans: an emerging class of conjugated oligomers for organic electronics. Angew. Chem. Int. Ed. 53, 2546–2555 (2014).
Gidron, O., Diskin-Posner, Y. & Bendikov, M. α-Oligofurans. J. Am. Chem. Soc. 132, 2148–2150 (2010).
Gidron, O., Shimon, L. J. W., Leitus, G. & Bendikov, M. Reactivity of long conjugated systems: selectivity of Diels–Alder cycloaddition in oligofurans. Org. Lett. 14, 502–505 (2012).
Gadakh, S., Shimon, L. J. W. & Gidron, O. Regioselective transformation of long π-conjugated backbones: from oligofurans to oligoarenes. Angew. Chem. Int. Ed 56, 13601–13605 (2017).
Dishi, O. & Gidron, O. Macrocyclic oligofurans: a computational study. J. Org. Chem. 83, 3119–3125 (2018).
Mulay, S. V. et al. A macrocyclic oligofuran: synthesis, solid state structure and electronic properties. Chem. Sci. 10, 8527–8532 (2019).
Dishi, O., Rahav, Y., Carmieli, R. & Gidron, O. A macrocyclic furan with accessible oxidation states: switching between aromatic and antiaromatic global ring currents. Chem. Eur. J. 28, e202202082 (2022).
Varni, A. J. et al. Design, synthesis, and properties of a six-membered oligofuran macrocycle. Org. Chem. Front. 8, 1775–1782 (2021).
Dishi, O., Malakar, P., Shimon, L. J. W., Ruhman, S. & Gidron, O. Ring size determines the conformation, global aromaticity and photophysical properties of macrocyclic oligofurans. Chem. Eur. J 27, 17794–17801 (2021).
Märkl, G., Stiegler, J., Kreitmeier, P. & Burgemeister, T. Neutrale aromatische Tetraepoxyannulene: Tetraepoxy[26]annulene(4.2.2.2) und Tetraepoxy[30]annulene(4.4.4.2) – Systeme mit hoher molekularer Dynamik. Helv. Chim. Acta 84, 2037–2050 (2001).
Märkl, G., Stiegler, J. & Kreitmeier, P. Tetraepoxy[32]annulene(4.4.4.4) und `Tetraoxa[30]porphyrin(4.4.4.4)’-Dikationen. Helv. Chim. Acta 84, 2022–2036 (2001).
Qiu, Y. et al. Synthesis of polyfuran and thiophene-furan alternating copolymers using catalyst-transfer polycondensation. ACS Macro Lett. 5, 332–336 (2016).
Jirásek, M., Rickhaus, M., Tejerina, L. & Anderson, H. L. Experimental and theoretical evidence for aromatic stabilization energy in large macrocycles. J. Am. Chem. Soc. 143, 2403–2412 (2021).
Eder, S. et al. Switching between local and global aromaticity in a conjugated macrocycle for high-performance organic sodium-ion battery anodes. Angew. Chem. Int. Ed 59, 12958–12964 (2020).
Minsky, A., Meyer, A. Y. & Rabinovitz, M. Paratropicity and antiaromaticity: role of the homo-lumo energy gap. Tetrahedron 41, 785–791 (1985).
Cho, S. et al. Defining spectroscopic features of heteroannulenic antiaromatic porphyrinoids. J. Phys. Chem. Lett. 1, 895–900 (2010).
Pierrefixe, S. C. A. H. & Bickelhaupt, F. M. Aromaticity: molecular-orbital picture of an intuitive concept. Chem. Eur. J. 13, 6321–6328 (2007).
Plasser, F. Exploitation of Baird aromaticity and Clar’s rule for tuning the triplet energies of polycyclic aromatic hydrocarbons. Chemistry 3, 532–549 (2021).
Chen, Z., Wannere, C. S., Corminboeuf, C., Puchta, R. & Schleyer, PvonR. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 105, 3842–3888 (2005).
Casademont-Reig, I., Guerrero-Avilés, R., Ramos-Cordoba, E., Torrent-Sucarrat, M. & Matito, E. How aromatic are molecular nanorings? The case of a six-porphyrin nanoring. Angew. Chem. Int. Ed 60, 24080–24088 (2021).
Deng, J.-R., Bradley, D., Jirásek, M., Anderson, H. L. & Peeks, M. D. Correspondence on “How Aromatic are Molecular Nanorings? The Case of a Six-Porphyrin Nanoring”. Angew. Chem. Int. Ed 134, e202201231 (2022).
Glöcklhofer, F. Concealed antiaromaticity. Preprint at https://doi.org/10.26434/chemrxiv-2023-hnl0w (2023).
Acknowledgements
This research was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant Agreement No. 850836, ERC Starting Grant “PolyHelix”). This research was supported by The Israel Science Foundation – FIRST Program (grant No. 1453/19).
Author information
Authors and Affiliations
Contributions
Y.R. and O.G. designed the experiments. Y.R. and S.K.R. synthesized the macrocycles, and performed the experiments. Y.R. and O.G. analyzed the experimental data. B.B. collected and analyzed the crystallographic data. Y.R., O.D., and O.G. conducted the computational study. Y.R. and O.G. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks Shohei Saito, Felix Plasser, and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahav, Y., Rajagopal, S.K., Dishi, O. et al. Alternating behavior in furan-acetylene macrocycles reveals the size-dependency of Hückel’s rule in neutral molecules. Commun Chem 6, 100 (2023). https://doi.org/10.1038/s42004-023-00902-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-023-00902-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.