Abstract
Super engineering plastics, high-performance thermoplastic resins such as polyetheretherketone, and polyphenylene sulfide have been utilized in industries, owing to their high thermal stability and mechanical strength. However, their robustness hinders their depolymerization to produce monomers and low-weight molecules. Presently, chemical recycling for most super engineering plastics remains relatively unexplored. Herein, we report the depolymerization of insoluble polyetheretherketone using sulfur nucleophiles via carbon–oxygen bond cleavages to form benzophenone dithiolate and hydroquinone. Treatment with organic halides converted only the former products to afford various dithiofunctionalized benzophenones. The depolymerization proceeded as a solid–liquid reaction in the initial phase. Therefore, this method was not affected by the shape of polyetheretherketone, e.g., pellets or films. Moreover, this depolymerization method was applicable to carbon- or glass fiber-enforced polyetheretherketone material. The depolymerized product, dithiofunctionalized benzophenones, could be converted into diiodobenzophenone, which was applicable to the polymerization.
Similar content being viewed by others
Introduction
For a long time, considerable effort has been dedicated to the development of technologies for reusing and recycling plastic materials1,2. Particularly, thermal and material recycling has been focused on and developed. In recent years, chemical recycling is becoming increasingly important as a way of chemically converting plastic materials into raw organic substrates and organic functional materials3,4,5,6,7,8,9,10,11,12. Gasification of plastic wastes is the typical protocol. Efforts have been devoted to the research and development of the monomerization of commodity plastics and engineering plastics such as polyethylene terephthalate and polycarbonate13,14,15. Recently, studies on stable engineering plastics such as polyamides and polyurethanes using transition metal catalysts have been actively conducted to provide low-weight molecules16,17. Additionally, the development of polymers containing degradable parts or functionalities to be converted into low-weight molecules is being actively pursued18,19,20,21,22,23,24,25.
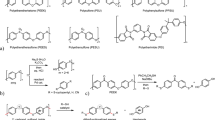
Thus, many studies on the chemical recycling of different plastics are ongoing. On the other hand, super engineering plastics such as polyetheretherketone (PEEK), polyphenylene sulfide (PPS), polysulfone (PSU), and polyethersulfone (PESU) known as commercially available high-performance thermoplastic resins exhibit high thermal stability and mechanical strength (Fig. 1a). Particularly, PEEK is a semicrystalline thermoplastic with outstanding characteristics such as chemical resistance, high melting point, and insolubility in organic solvents, in addition to the aforementioned thermal stability and high mechanical strength26. However, these advantages hinder its depolymerization to produce monomers and related low-weight molecules. Currently, the existing depolymerization studies involve only PPS and PESU (Fig. 1b)27,28,29,30,31,32, indicating that there are no generic depolymerization methods for these resins. This will constitute a significant environmental burden in the future. Additionally, discarding such high-priced products would result in a significant economic loss. Thus, versatile depolymerization methods for super engineering plastics are in high demand. To overcome this challenge, the development of reaction methodologies to approach insoluble chemicals and cleave stable carbon–oxygen bonds is required. This is because the existing reaction formats with small soluble molecules are not directly applicable. Actually, based on reported conditions33,34, amination reactions via carbon–oxygen bond cleavage were inapplicable to PEEK (see Supplementary Fig. 1).
a Examples of super engineering plastics. b Reported depolymerization of PPS or PESU to produce low-weight molecules. c This work: PEEK depolymerization using sulfur nucleophiles to afford two anionic monomer intermediates, followed by selective functionalization with organic halides produced dithiofunctionalized benzophenones and hydroquinone. NMP, N-methyl-2-pyrrolidone; SingaCycle-A1, chloro[[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](N,N-dimethylbenzylamine)palladium(II)]; IcHex·HCl, 1,3-dicyclohexylimidazolium chloride; cod, 1,5-cyclooctadiene; dcype, 1,2-bis(dicyclohexylphosphino)ethane; R–X, organic halide.
The ideal depolymerization is to regenerate the original monomer, i.e., halogenated monomers. However, this is thermodynamically impossible. Thus, we focused on sulfur nucleophiles. Sulfur can be introduced into various organic compounds, and the sulfur functional group can be converted into sulfonium salts as useful eliminating groups35,36. Using a transition metal catalyst, both the sulfonium group and the original sulfur functional group can be used as leaving groups to form new bonds37. Therefore, if super engineering plastics can be depolymerized and functionalized using sulfur reactants, the prevailing problem can be solved. It is known that a thiolate anion, an active form of the sulfur reactant, can interact with electron-deficient arenes to form an electron-donor–acceptor (EDA) complex38,39,40. We expected that this phenomenon would be effective for the interaction of the main chain of the insoluble PEEK molecular surface, and promote the cleavage of the main chain.
Herein, we report the depolymerization of insoluble PEEK (the robust super engineering plastic) using sulfur nucleophiles to afford two monomers without collapsing the molecular architecture of the main chain. This depolymerization comprised a carbon–oxygen main-chain cleavage and an aryl thiolate generation sequence. The sulfur nucleophiles became a source of sulfur anions toward a benzophenone monomer block (Fig. 1c). The subsequent introduction of carbonaceous groups on sulfur with organic halides yielded dithiofunctionalized benzophenone, with the remaining hydroquinone monomer intact. Using 2-phenylethanethiol as the sulfur nucleophile, we successfully achieved a high yield of two monomers. The utility of this unique methodology is that it was not affected by the forms of the PEEK and additives such as glass fibers.
Results and discussion
Optimization of the reaction conditions
The bond dissociation energy of carbon–oxygen bonds is higher than that of carbon–sulfur bonds41. In fact, nucleophilic etherification of methylthio-substituted benzonitriles was reported42. Therefore, the substitution reaction from oxygen to sulfur on carbon appeared difficult. However, we evaluated the efficacy of the exchange reaction from oxygen to sulfur functional groups applying model substrates, 4-(4-phenoxyphenyl)benzophenone and sodium sulfide, using density functional theory (DFT) calculations (See supplementary Fig. 15). The calculated relative free Gibbs energy was −91.7 kcal/mol between these substrates and the products, 4-sodium benzophenone 4-thiolate and sodium 4-phenoxyphenolate as a thermodynamically much stable product, showing that this process was thermodynamically favorable.
Thus, we applied 2 equiv. of sodium sulfide for the depolymerization of PEEK as a powder (Mw ~20,800 and Mm ~10,300) with 1,3-dimethyl-2-imidazolidinone (DMI) as a solvent at 150 °C for 17 h, followed by quenching with iodomethane. In fact that PEEK is insoluble in most organic solvents even at high temperatures, we confirmed that PEEK did not dissolve and swell in DMI at 150 °C. However, depolymerization proceeded to afford the desired monomer product, 4,4′-dimethylthiobenzophenone (5), in a small yield (10%), accompanied by comonomer 4 (44% yield) (Fig. 2a). This result showed that the carbon–oxygen bond on the benzophenone unit was selectively cleaved, and the intermediate comonomer, 4-[4-{4-NaSC6H4C(O)}C6H4O]C6H4ONa (2), and monomer, (4-NaSC6H4)2CO (3) were formed in situ. Following methylation occurred at sodium thiolates in preference to sodium arylates. The aryloxylate group (─OC6H4ONa) in 2 was not a suitable eliminating group; therefore, the formation of the dithiolate 3 was suppressed (see Supplementary Table 1).
a Reaction of PEEK with sodium sulfide followed by the treatment with iodomethane. b Development of PEEK depolymerization. Yields were determined via gas chromatography and nuclear magnetic resonance (NMR). The numbers in parentheses are isolated yields. c Cleavage of carbon–oxygen bond on the benzophenone unit by sodium alkylthiolates to form para-alkylthio-benzophenone units, followed by the nucleophilic attack of the sodium alkylthiolates to the alkyl group on sulfur. d Exchange between intermediate sodium arylates and the thiols to provide aryl alcohols and sodium thiolates. e Time-dependent conversion for the reaction of PEEK powder (1 equiv. relative to the molecular weight of the monomer), PhCH2CH2SH (4 equiv.), NaOt-Bu (3 equiv.), and DMI at 150 °C, followed by quenching with iodomethane at 100 °C. f Depolymerization of PEEK pellets or films. a4-Hexylthio-4′-methylthio-benzophenone was observed in 38% yield. bN,N-dimethylacetamide was used as a solvent. cHydroquinone (7) was obtained in high yield (>95%) determined by 1H NMR analysis. DMAc N,N-dimethylacetamide.
In considering approaches to increase the yield of 5, we focused on a more reactive aliphatic thiolate nucleophile for the formation of the dithiolate 3. This thiolate nucleophile has a dual role, exchanging the aryloxy group on the benzophenone unit for an alkylthio group and eliminating the alkyl group from the introduced alkylthio group43,44 to furnish a sodium thiolate (Fig. 2c). As a result, the use of 2-phenylethanethiol (4 equiv.) with sodium tert-butoxide (NaOt-Bu) (4 equiv.) was effective, affording 5 in 88% yield (Fig. 2b, Entry 1). This depolymerization proceeded smoothly in the air (see Supplementary Table 4, Entry 2). On the other hand, when n-hexanethiol was used, the depolymerization itself proceeded smoothly, but gave 5 in a lower yield (51%) and an intermediate, 4-hexylthio-4′-methylthio-benzophenone (6) in 38% yield (Fig. 2b, Entry 2). In contrast, benzylthiol did not undergo complete depolymerization, leaving 4 at a moderate yield (Fig. 2b, Entry 3). Other conditions using n-hexanethiol or benzylthols were screened but 5 was not obtained in higher yield with 2-phenylethanethiol (see Supplementary Tables 2 and 3).
Thus, 2-phenylethanethiol exhibited good performance for this depolymerization of PEEK, but 4 remained at a low yield under the conditions. We assumed that the in situ-generated ─OC6H4ONa group can activate thiols and be transformed into an aryl alcohol group (─OC6H4OH) as a better-eliminating group (Fig. 2d and Supplementary Fig. 16). In fact, the arylate group (e.g., pKa of PhOH in DMSO: 18.0)45 exhibits sufficient basicity to activate the aliphatic thiols (e.g., pKa of n-BuSH in DMSO: 17.0)46. This was supported using DFT calculations (see Supplementary Fig. 17). Successfully, the depolymerization with 3 equiv. of NaOt-Bu led to the production of 5 in high yield (84% isolated yield) (Fig. 2b, Entry 4), whereas changing to 2 equiv. of the base diminished the yield of 5, probably due to decreased reaction rate (Fig. 2b, Entry 5). Using NaOH as a commodity and cheap base instead of NaOt-Bu, the yield of 5 was maintained (Fig. 2b, Entry 6). Finally, this depolymerization of PEEK in N,N-dimethylacetamide afforded 5 in 93% isolated yield while regenerating hydroquinone (7) in more than 95% NMR yield (Fig. 2b, Entry 7). At this time, di(2-phenylethyl)sulfide and styrene was formed in 94% and 4% yields based on the amount of the thiol, respectively, which can be reconverted into the original thiol47,48. Notably, at the ambient temperature (30 °C), no monomer and comonomer products were observed (see Supplementary Table 4, Entry 15). Other thiols, such as 2-mercaptoethanol and 2-ethylhexyl-3-mercaptopropanoate containing easily removable carbonaceous groups49,50 reduced the yields of 5 (see Supplementary Table 3, Entries 16–19).
To examine the efficiency of this depolymerization, we monitored the reaction of PEEK powder with 2-phenylethanethiol under certain conditions shown in Fig. 2b, Entry 4. Unexpectedly, the depolymerization of the insoluble PEEK was practically completed after 1 h, affording 5 and 4-phenethylthio-4′-methylthio-benzophenone (8) in 25% and 60% yields, respectively, with small amounts of comonomers (Fig. 2e). Intermediate 8 was gradually converted into 5. This showed that 2-phenylethyl thiolate is effective for the facile depolymerization of PEEK solids, giving the monomer intermediates speedily. In case of using Na2S, the cleavage of the main chain of PEEK itself was indicated to proceed rapidity (see Supplementary Fig. 8 and 9). With this observation, we expected that this method is insulated from the influence of the PEEK form. In fact, both pellet (average Mw ~20,800; average Mn ~10,300; mean particle size, 80 microns) and film (thickness, 0.025 mm) forms of PEEK smoothly underwent depolymerization sequence to afford 5 and 7 in excellent yields (Fig. 2f). It is noteworthy that the PEEK pellets did not dissolve and divide into small parts but were continuously dwindling during this depolymerization (see Supplementary Table 5 and Fig. 7). This showed that the surface moieties of the PEEK materials reacted with the thiolate without dissolving.
Substrate scope
To demonstrate the scope of this one-pot protocol, we examined various electrophiles after the depolymerization of PEEK powder under optimal conditions (Fig. 2b, Entry 7). As shown in Fig. 3, various alkyl halides were applicable in this sequence, and corresponding benzophenone-derived monomer products 9-22 were isolated at excellent yields. For example, 2-bromoethanol underwent the alkylation to form 17 with two hydroxy groups at a good yield51,52, showing that the hydroxy group did not interfere with this process. 2-(Bromomethyl)oxirane was uneventfully applicable to this process to form 18, which is a sulfur analog of a benzophenone-based monomer of epoxy resins53,54,55,56. Notably, the depolymerization/functionalization sequence could be easily performed on a gram scale. The use of 1-bromo-3,7-dimethyloctane was selected in the gram scale sequence, which afforded 19 in 81% yield. In addition to alkyl halides, an acid chloride was applicable in this sequence to form a corresponding monomer product, 20 in 81% yield. Further treatment with hydrogen chloride after depolymerization afforded 4,4’-dimercaptobenzophenone (21)57. This one-pot depolymerization/functionalization method was applicable in the three-step sequence. After treatment with 2-bromoethanol, in situ formed 17 was subjected to esterification with methacryloyl chloride, and the corresponding product 22 reported as a monomer for high refractive index resin was easily obtained51,52.
Reaction conditions: (i) PEEK powder (0.3 mmol relative to the molecular weight of the monomer), PhCH2CH2SH (1.2 mmol), NaOt-Bu (0.9 mmol), DMAc (0.6 mL), 150 °C, 20–22 h. (ii) Electrophile (0.9 mmol) under conditions in parentheses. For each compound, the isolated yield is given in percentage. aPEEK powder (4.0 mmol relative to the molecular weight of the monomer), PhCH2CH2SH (16 mmol), NaOt-Bu (12 mmol), DMAc (8 mL), 150 °C, 22 h. (ii) 1-bromo-3,7-dimethyloctane (12 mmol), 100 °C, 2 h. baq. HCl (2 M, 2.0 mL). cNMR yield. dAfter step (ii), methacryloyl chloride (1.8 mmol), triethylamine (3.6 mmol), and dichloromethane (0.6 mL) was added to the mixture, which was stirred at room temperature for 64 h.
Selective depolymerization of PEEK
Given that the resin frequently contains an additional agent, we investigated the depolymerization of PEEK powder in the presence of glass fibers. As shown in Table 1, Entry 1, the depolymerization proceeded smoothly to form 5 and hydroquinone (7) in 94 and 92% yields, respectively. Additionally, other commodity polymers such as polypropylene, polystyrene, and Nylon 6 did not interfere with the depolymerization, affording 5 and 7 in good yields (Table 1, Entries 2−4). Thereafter, carbon or glass fiber-reinforced PEEK material was used for the depolymerization experiment. A roughly ground carbon fiber (30 wt%) reinforced PEEK material was subjected to a reaction with 2-phenylethanethiol and NaOt-Bu, followed by treatment with iodomethane, affording 5 and 7 in good yields (Table 1, Entry 5). Similarly, a roughly ground PEEK compound including glass fiber (30 wt%) was examined under the same conditions, also resulting in 5 and 7 (Table 1, Entry 6).
Experimental mechanistic studies
As previously mentioned, 2-phenylethanethiol is a promising depolymerization reagent for PEEK. To understand this efficiency, we examined the reaction of 4,4′-diphenoxy-benzophenone (23) as a PEEK model compound with 2 equiv. of selected thiols and NaOt-Bu at 150 °C for 1 h. First, the reaction using n-hexanethiol formed di(n-hexylthio)-substituted benzophenone (9) as a simple substitution product and following dehexylated/monomethylated 6 in 70% and 22% yields, respectively (Table 2, Entry 1). Benzyl mercaptan afforded dimethylated product 5 in 17% yield with the observation of dibenzyl sulfide (32% based on the amount of the thiol) (Table 2, Entry 2). These results were due to the stability toward nucleophilic attack; hexyl group is robust, whereas the benzyl group is weak. In contrast, the reaction using 2-phenylethanethiol furnished a mixture of monomethylated 8, 5, and styrene in 67, 26, and 37% yields, respectively, without the generation of bis(2-phenylethyl)sulfide (Table 2, Entry 3). For longer reaction time, the yield of 5 was increased to 70% (see Supplementary Fig. 2). At room temperature, this reaction took place to afford 11 selectively in 95% yield (see Supplementary Fig. 3). The generation of styrene means that base-mediated elimination from the phenethyl group on 8 and 11 occurs gradually to provide sodium thiolates58. Thus, these results show that 2-phenylethanethiol can lead to the formation of (4-NaSC6H4)2CO (3) effectively via the smooth carbon–oxygen bond-cleaving substitution followed by two types of dealkylation; second nucleophilic substitution (Fig. 2c) and styrene elimination. In fact, we confirmed that these dealkylations proceed (see Supplementary Fig. 6).
As mentioned above, the present depolymerization was initiated on the surface of the PEEK materials using the thiolate. This suggestion was supported by the S K-edge X-ray absorption near-edge structures (XANES) analysis of degradation samples prepared by the reaction of PEEK powder with sodium sulfide at the early stage (see Supplementary Figs. 10, 11). A thiolate anion and an electron-deficient arene such as benzophenone are known to associate to form an EDA complex (see Supplementary Figs. 12–14)59. We expected that this EDA complex would retain the thiolate anion on the PEEK surface and promote the surface carbon–oxygen bond cleavage, maybe via the SNAr or SRA160,61,62 mechanism. In the case of SRA1, radical-chain and nonchain mechanisms are proposed. In this regard, this PEEK depolymerization provided hydroquinone, which is known as an inhibitor of the generation of free radicals, suggesting that free radicals were not generated during the depolymerization (see Supplementary Fig. 4,5). In addition, 4 equiv. of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO), as a radical scavenger, did not affect the depolymerization for 3 h and methylation, giving 5 and 8 in good yields whereas the yield of 5 was lower than the TEMPO-free conditions (Fig. 4). These results might at least allow us to exclude the possibility of a radical pathway under the depolymerization step by alkylthiolates.
Proposed mechanism
Based on the experimental results, we show a proposed depolymerization pathway in Fig. 5. The benzophenone unit at the PEEK surface and an organic thiolate derived from the corresponding thiol with NaOt-Bu first associate to form an EDA complex. The sulfur center of the thiolate then attacks the para-carbon bound to oxygen via the SNAr or nonchain SRA1 mechanism, and the aryloxy anion is released to complete the formation of the carbon–sulfur bond. The generated aryloxy anion or NaOt-Bu activates the thiol to form the organic thiolate, which undergoes an SN2 reaction with the generated alkyl aryl sulfide to produce the benzophenone thiolate moiety and a dialkylsulfide. In the case using 2-phenylethanethiol, the base-mediated elimination of styrene from 2-phenylethylthio group also proceeds sluggishly to form the thiolates and styrene. This series of processes repeatedly occurred to finally obtain the benzophenone dithiolate 3.
Utility of products
Alkylthio groups can be converted into reactive sulfonium groups. We confirmed the methylation of 5 using methyl trifluoromethane sulfonate in 1,2-dichloroethane at 60 °C, based on a reported method63, and obtained the benzophenone 4,4′-bis(dimethylsulfonium) salt, 24 in excellent yield (Fig. 6a). Afterward, we attempted substitution to iodine. Based on a reported nickel catalytic method developed by Yorimitsu64, 24 was converted into 4,4′-diiodobenzophenone (25) as an active form of various substitution reactions65,66,67. In fact, 25 was applicable to the polymerization with 2,2′-bis(4-hydroxyphenyl)propane (26) under copper-catalyzed conditions68 to give the corresponding copolymer 2769,70,71,72 with Mw = 24,039 and PDI = 3.49 in 87% yield after reprecipitation (Fig. 6b). We also examined the polymerization using molecules obtained by the present depolymerization (see Supplementary pages S22–S24). The reaction of 4,4′-dimercaptobenzophenone (21) with nonanedioyl dichloride (28) underwent in chloroform under reflux73 to form a polythioester 29 with Mw = 49,641 and PDI = 2.04 in 94% yield (Fig. 6c).
a Transformation of 4,4′-di(methylthio)benzophenone (5) to benzophenone 4,4′-bis(dimethylsulfonium) salt 24 followed by iodination to form 4,4′-diiodobenzophenone (25). b Polymerization of 25 with bis-phenol A (26). c Polymerization of 21 with nonanedioyl dichloride (28). MeOTf methyl trifluoromethane sulfonate, glyme dimethoxyethane; Neocuproine, 2,9-dimethyl-1,10-phenanthroline, DMF N,N-dimethylformamide.
Conclusion
In this study, we demonstrated that insoluble PEEK, as a robust super engineering plastic, can be depolymerized for the formation of monomer units. In this process, the 2-phenylethanethiolate reagent was effective for the depolymerization of PEEK, followed by treatment with organic halides to furnish dithiofunctionalized benzophenones and hydroquinone in high yields. A series of organic halides were applied after the depolymerization, providing various dithiofunctionalized benzophenones. The products can be converted into a bis(sulfonium) salt and diiodobenzophenone as an active form of various substitution reactions. Several produced monomer units were applied to polymerization reactions. The depolymerization proceeded as a solid–liquid reaction in the initial phase. Therefore, the present depolymerization method was applicable to various forms of pure PEEK, such as powder, pellet, and film. Moreover, glass or carbon fiber-reinforced PEEK materials were utilized for this depolymerization. This development opens up the application of PEEK in chemical recycling and highlights the potential of this strategy to unlock the depolymerization of other highly stable resins. Further efforts are underway to exploit the catalytic methodology for the depolymerization of PEEK and to expand the scope of other super engineering plastics and related robust polymer materials.
Methods
General procedure for depolymerization of PEEK
N,N-Dimethylacetamide (0.60 mL) and 2-phenylethanethiol (167 mg, 1.21 mmol) were added to a mixture of PEEK powder (86.4 mg, 0.300 mmol relative to the molecular weight of the monomer) and sodium tert-butoxide (86.5 mg, 0.900 mmol) in a 3 mL vial in an argon atmosphere. The mixture was stirred at 150 °C for 20 h. After the liquid mixture cooled to room temperature, iodomethane (128 mg, 0.900 mmol) was added and stirred at 100 °C for 1 h. After ethyl acetate (1.5 mL) was added, the mixture was washed with aqueous HCl (2 M, 1 mL), water, and brine. At this time, the mixture was analyzed by 1H NMR to determine the yields of hydroquinone (7) (>95%) and styrene (4%). The extracted organic layer was dried over Mg2SO4 and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (hexane/ethyl acetate, 96:4 to 7:3) to afford bis(4-(methylthio)phenyl)methanone (5) (93%, 75.9 mg).
General information. See Supplementary Methods, general information (page S3).
Chemicals. See Supplementary Methods, chemicals (page S4).
NMR charts. See Supplementary Data 1, NMR spectra of obtained chemicals.
GPC charts. See Supplementary Data 1, GPC charts.
Data availability
The data obtained in this study are available within this article and its supplementary information and are also from the corresponding authors upon reasonable request. Original 1H and 13C spectra of the compounds obtained in this manuscript are available in Supplementary Data 1. The computed energy values and coordinates are available in Supplementary Data. 2.
References
Ignatyev, I. A., Thielemans, W. & Beke, B. V. Recycling of polymers: a review. ChemSusChem 7, 1579–1593 (2014).
Stadler, B. M., Wulf, C., Werner, T., Tin, S. & de Vries, J. G. Catalytic approaches to monomers for polymers based on renewables. ACS Catal. 9, 8012–8067 (2019).
Hong, M. & Chen, E. Y.-X. Chemically recyclable polymers: a circular economy approach to sustainability. Green. Chem. 19, 3692–3706 (2017).
Rahimi, A. & García, J. M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 1, 0046 (2017).
Lu, Z.-B., Liu, Y. & Zhou, H. Learning nature: recyclable monomers and polymers. Chem. Eur. J. 24, 11255–11266 (2018).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Kim, J. G. Chemical recycling of poly(bisphenol A carbonate). Polym. Chem. 1, 4830–4849 (2020).
Payne, J. & Jones, M. D. The chemical recycling of polyesters for a circular plastics economy: challenges and emerging opportunities. ChemSusChem 14, 4041–4070 (2021).
Chen, H., Wan, K., Zhang, Y. & Wang, Y. Waste to wealth: chemical recycling and chemical upcycling of waste plastics for a great future. ChemSusChem 14, 4123–4136 (2021).
Liguori, F., Moreno-Marrodán, C. & Barbaro, P. Valorisation of plastic waste via metal-catalysed depolymerization. Beilstein J. Org. Chem. 17, 589–621 (2021).
Kosloski-Oh, S. C., Wood, Z. A., Manjarrez, Y., de los Rios, J. P. & Fieser, M. E. Catalytic methods for chemical recycling or upcycling of commercial polymers. Mater. Horiz. 8, 1084–1129 (2021).
Xu, G. & Wang, Q. Chemically recyclable polymer materials: polymerization and depolymerization cycles. Green. Chem. 24, 2321–2346 (2022).
Huang, W. et al. Degradation of polycarbonate to produce bisphenol A catalyzed by imidazolium-based DESs under metal-and solvent-free conditions. RSC Adv. 11, 1595–1604 (2021).
Tanaka, S., Sato, J. & Nakajima, Y. Capturing ethylene glycol with dimethyl carbonate towards depolymerisation of polyethylene terephthalate at ambient temperature. Green. Chem. 23, 9412–9416 (2021).
Lu, H. et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 604, 662–667 (2022).
Kumar, A. et al. Hydrogenative depolymerization of nylons. J. Am. Chem. Soc. 142, 14267–14275 (2020).
Zhou, W. et al. Depolymerization of technical-grade polyamide 66 and polyurethane materials through hydrogenation. ChemSusChem 14, 4176–4180 (2021).
Christensen, P. R., Scheuermann, A. M., Loeffler, K. E. & Helms, B. A. Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds. Nat. Chem. 11, 442–448 (2019).
Beromi, M. M. et al. Iron-catalysed synthesis and chemical recycling of telechelic 1,3-enchained oligocyclobutanes. Nat. Chem. 13, 156–162 (2021).
Sathe, D. et al. Olefin metathesis-based chemically recyclable polymers enabled by fused-ring monomers. Nat. Chem. 13, 743–750 (2021).
Nguyen, S. T., McLoughlin, E. A., Cox, J. H., Fors, B. P. & Knowles, R. R. Depolymerization of hydroxylated polymers via light-driven C−C bond cleavage. J. Am. Chem. Soc. 143, 12268–12277 (2021).
Huang, B. et al. Backbone-photodegradable polymers by incorporating acylsilane monomers via ring-opening metathesis polymerization. J. Am. Chem. Soc. 143, 17920–17925 (2021).
Chen, H., Shi, Z., Hsu, T.-G. & Wang, J. Overcoming the low driving force in forming depolymerizable polymers through monomer isomerization. Angew. Chem. Int. Ed. 60, 25493–25498 (2021).
Zhou, J., Sathe, D. & Wang, J. Understanding the structure−polymerization thermodynamics relationships of fused-ring cyclooctenes for developing chemically recyclable polymers. J. Am. Chem. Soc. 144, 928–934 (2022).
Fouilloux, H., Rager, M.-N., Ríos, P., Conejero, S. & Thomas, C. M. Highly efficient synthesis of poly(silylether)s: access to degradable polymers from renewable resources. Angew. Chem. Int. Ed. 61, e202113443 (2022).
Rose, J. B. & Staniland, P. A. Thermoplasticaromatic polyetherketones. US Patent 4,320,224 (1982).
Yu, Z. L., Miao, G. X., Wu, Q. & Chen, Y. Synthesis of thiol- and carboxyl-terminated poly(p-phenylene sulfide) oligomers. Macromol. Chem. Phys. 197, 4061–4068 (1996).
Wang, S. J., Bian, S. G., Yan, H., Xiao, M. & Meng, Y. Z. Novel synthesis of macrocyclic disulfides from poly(phenylene sulfide) by depolymerization reaction. J. Appl. Poly. Sci. 110, 4049–4054 (2008).
Lian, Z., Bhawal, B. N., Yu, P. & Morandi, B. Palladium-catalyzed carbon-sulfur or carbon-phosphorus bond metathesis by reversible arylation. Science 356, 1059–1063 (2017).
Minami, Y. et al. Catalytic reductive cleavage of poly(phenylene sulfide) using a hydrosilane. Synthesis 53, 3351–3354 (2021).
Delcaillau, T., Woenckhaus-Alvarez, A. & Morandi, B. Nickel-catalyzed cyanation of aryl thioethers. Org. Lett. 23, 7018–7022 (2021).
Yoshida, H. & Fukunaka, T. Method for producing raw material monomer of polyether sulfone. Japanese Patent JP2013249324 (2013).
Shigeno, M., Hayashi, K., Nozawa-Kumada, K. & Kondo, Y. Organic superbase t‑Bu-P4 catalyzes amination of methoxy(hetero)arenes. Org. Lett. 21, 5505–5508 (2019).
Tobisu, M., Takahira, T., Morioka, T. & Chatani, N. Nickel-catalyzed alkylative cross-coupling of anisoles with Grignard reagents via C−O bond activation. J. Am. Chem. Soc. 138, 6711–6714 (2016).
Tian, Z.-Y., Hu, Y.-T., Teng, H.-B. & Zhang, C.-P. Application of arylsulfonium salts as arylation reagents. Tetrahedron Lett. 59, 299–309 (2018).
Yorimitsu, H. Catalytic transformations of sulfonium salts via C-S bond activation. Chem. Rec. 21, 3356–3369 (2021).
Otsuka, S., Nogi, K. & Yorimitsu, H. C-S bond activation. Top. Curr. Chem. 376, 199–237 (2018).
Liu, B., Lim, C.-H. & Miyake, G. M. Visible-light-promoted C–S cross-coupling via intermolecular charge transfer. J. Am. Chem. Soc. 139, 13616–13619 (2017).
Crisenza, G. E. M., Mazzarella, D. & Melchiorre, P. Synthetic methods driven by the photoactivity of electron donor–acceptor complexes. J. Am. Chem. Soc. 142, 5461–5476 (2020).
Li, H. et al. Polysulfide anions as visible light photoredox catalysts for aryl cross-couplings. J. Am. Chem. Soc. 143, 481–487 (2021).
Aylward, G. & Findlay, T. (eds) SI Chemical Data 6th edn (John Wiley & Sons, 2008).
Wang, X. et al. Nucleophilic amination and etherification of aryl alkyl thioethers. Org. Lett. 20, 4749–4753 (2018).
Pinchart, A., Dallaire, C. & Gingras, M. Functionalized p-phenylene sulfides synthesis of new molecular wires. Tetrahedron Lett. 39, 543–546 (1998).
Pinchart, A., Dallaire, C., Bierbeek, A. V. & Gingras, M. Efficient formation of aromatic thiols from thiomethylated precursors. Tetrahedron Lett. 40, 5479–5482 (1999).
Bordwell, F. G., McCallum, R. J. & Olmstead, W. N. Acidities and hydrogen bonding of phenols in dimethyl sulfoxide. J. Org. Chem. 49, 1424–1427 (1984).
Bordwell, F. G. & Hughes, D. L. Thiol acidities and thiolate ion reactivities toward butyl chloride in dimethyl sulfoxide solution. The question of curvature in Broensted plots. J. Org. Chem. 47, 3224–3232 (1982).
Yu, Z. & Verkade, J. G. Mild and efficient desulfurization of alkyl sulfides with sodium. Tetrahedron Lett. 39, 2671–2674 (1998).
Yi, X. et al. Method for preparing a mercaptan compound by Michael addition. Chinese Patent CN201410507778 (2015).
Liu, Y., Kim, J., Seo, H., Park, S. & Chae, J. Copper(II)-catalyzed single-step synthesis of aryl thiols from aryl halides and 1,2-ethanedithiol. Adv. Synth. Catal. 357, 2205–2212 (2015).
Becht, J.-M., Wagner, A. & Mioskowski, C. Facile introduction of SH group on aromatic substrates via electrophilic substitution reactions. J. Org. Chem. 68, 5758–5761 (2003).
Tamura, Y. & Hayakawa, S. Method for producing sulfur-containing acrylic compound. Japanese Patent JP2001172253 (2001).
Tamura, Y. & Hayakawa, S. Method for producing sulfur-containing acrylic compound. Japanese Patent JP2001172255 (2001).
Vogel, W. et al. Synthesis and characterisation of new sulphur-containing epoxy networks. High. Perform. Poly 26, 420–435 (2014).
Jiang, X., Luo, X. & Yin, J. Polymeric photoinitiators containing in-chain benzophenone and coinitiators amine: Effect of the structure of coinitiator amine on photopolymerization. J. Photo Photo A. Chem. 174, 165–170 (2005).
Podkościelna, B., Gawdzik, B. & Bartnicki, A. Use of a new methacrylic monomer, 4,40-Di(2-hydroxy-3-methacryloyloxypropoxy)benzophenone, in the synthesis of porous microspheres. J. Poly. Sci. Part A Poly. Chem. 44, 7014–7026 (2006).
Zhang, C. et al. Inspired by elastomers: fabrication of hydrogels with tunable properties and re-shaping ability via photo-crosslinking at a macromolecular level. Polym. Chem. 8, 1824–1832 (2017).
Wu, Z., Yan, G., Lu, J., Zhang, G. & Yang, J. Thermal plastic and optical transparent polyimide derived from isophorone diamine and sulfhydryl compounds. Ind. Eng. Chem. Res. 58, 6992–7000 (2019).
Abidi, N. & Schmink, J. R. Synthesis of disubstituted dithioethers: tert-Butoxide promoted elimination/ring opening of 1,3-dithianes followed by palladium-catalyzed C−S bond formation. J. Org. Chem. 80, 4123–4131 (2015).
Yang, Z. et al. Synthetic reactions driven by electron-donor–acceptor (EDA) complexes. Beilstein J. Org. Chem. 17, 771–799 (2021).
Rossi, R. A., Pierini, A. B. & Peñéñory, A. B. Nucleophilic substitution reactions by electron transfer. Chem. Rev. 103, 71–167 (2003).
Zhang, X. M., Yang, D. L. & Liu, Y. C. Effects of electron acceptors and radical scavengers on nonchain radical nucleophilic substitution reactions. J. Org. Chem. 58, 224–227 (1993).
Zhang, X. M., Yang, D. L., Jia, X. Q. & Liu, Y. C. Kinetic and mechanistic studies of the nonchain radical nucleophilic substitution reactions. J. Org. Chem. 58, 7350–7354 (1993).
Minami, H., Otsuka, S., Nogi, K. & Yorimitsu, H. Palladium-catalyzed borylation of aryl sulfoniums with diborons. ACS Catal. 8, 579–583 (2018).
Yamada, K., Yanagi, T. & Yorimitsu, H. Generation of organozinc reagents from arylsulfonium salts using a nickel catalyst and zinc dust. Org. Lett. 22, 9712–9718 (2020).
Albrecht, K., Kasai, Y., Kimoto, A. & Yamamoto, K. The synthesis and properties of carbazole-phenylazomethine double layer-type dendrimers. Macromolecules 41, 3793–3800 (2008).
Albrecht, K. & Yamamoto, K. Dendritic structure having a potential gradient: new synthesis and properties of carbazole dendrimers. J. Am. Chem. Soc. 131, 2244–2251 (2009).
Sharmoukh, W., Ko, K. C., Noh, C., Lee, K. C. & Son, S. U. Designed synthesis of multi-electrochromic systems bearing diaryl ketone and isophthalates. J. Org. Chem. 75, 6708–6711 (2010).
Drapeau, M. P., Ollevier, T. & Taillefer, M. On the frontier between nucleophilic aromatic substitution and catalysis. Chem. Eur. J. 20, 5231–5236 (2014).
Salunke, A. K., Sharma, M., Kute, V. & Banerjee, S. Synthesis of novel copoly(ether ether ketones): property evaluation and microstructure analysis by NMR. J. Appl. Poly. Sci. 96, 1292–1305 (2005).
Kang, K. & Kim, D. Comparison of proton conducting polymer electrolyte membranes prepared from multi-block and random copolymers based on poly(arylene ether ketone). J. Power Sources 281, 146–157 (2015).
Kang, K. & Kim, D. Pendant dual-sulfonated poly(arylene ether ketone) multi-block copolymer membranes for enhanced proton conductivity at reduced water swelling. J. Membr. Sci. 578, 103–110 (2019).
Alentiev, A. et al. Structure-property relationship on the example of gas separation characteristics of poly(arylene ether ketone)s and poly(diphenylene phtalide). Membranes 11, 677 (2021).
Fukuda, N. Suzuki, M., Hirano, H., Agari, Y. & Kadota, J. Epoxy resin composition. Japanese Patent JP 2012144622 (2011).
Acknowledgements
This work was supported financially by PRESTO (JPMJPR21N9 to Y.M.) from the JST, Fujimori Science and Technology Foundation, Iketani Science and Technology Foundation, Grants-in-Aid for Scientific Research (C) (19K05481 to Y.M.) from the JSPS, and Department of Materials and Chemistry, AIST. Y.M., N.M., and Y.N. also acknowledge the DIC Corporation. Y.M. thanks JST, ERATO (JPMJER2103), and Prof. Kyoko Nozaki and her lab members for discussions on this project. Y.M. thanks Dr. Shinji Tanaka for the solid-state NMR analysis. A part of this work was performed under the approval of the Photon Factory Program Advisory Committee (Proposal No. 2021PF-G021). We would like to thank Dr. Shinji Tanaka for his contribution to the analysis of the polymer products and Ms. Tomoo Tsuyuki, Ms. Risa Kawato, and Mr. Yuki Inagaki for their kind assistance in experiments. We dedicated ourselves to Professor Shigeru Yamago on the occasion of his 60th birthday.
Author information
Authors and Affiliations
Contributions
Y.M. conceived the idea and designed the whole experiment with N.M. N.M. also worked with Y.M. to plan the conversion of the products and polymer synthesis. Y.M., N.M., and M.S. performed the experiments. Y.T. carried out XANES analysis. R.W. carried out analytical pyrolysis experiments. Y.M., N.M., Y.T., R.W., and Y.N. contributed to writing the manuscript and participated in data analyses and discussions. Y.M., N.M., and Y.N. revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks Junpeng Wang and the other, anonymous, reviewer for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Minami, Y., Matsuyama, N., Takeichi, Y. et al. Depolymerization of robust polyetheretherketone to regenerate monomer units using sulfur reagents. Commun Chem 6, 14 (2023). https://doi.org/10.1038/s42004-023-00814-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-023-00814-8
This article is cited by
-
Catalytic thiolation-depolymerization-like decomposition of oxyphenylene-type super engineering plastics via selective carbon–oxygen main chain cleavages
Communications Chemistry (2024)
-
Organomediated polymerization
Communications Chemistry (2024)
-
Alcoholysis of oxyphenylene-based super engineering plastics mediated by readily available bases
Polymer Journal (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.