Abstract
In the biological magnetic compass, blue-light photoreceptor protein of cryptochrome is thought to conduct the sensing of the Earth’s magnetic field by photoinduced sequential long-range charge-separation (CS) through a cascade of tryptophan residues, WA(H), WB(H) and WC(H). Mechanism of generating the weak-field sensitive radical pair (RP) is poorly understood because geometries, electronic couplings and their modulations by molecular motion have not been investigated in the secondary CS states generated prior to the terminal RP states. In this study, water dynamics control of the electronic coupling is revealed to be a key concept for sensing the direction of weak magnetic field. Geometry and exchange coupling (singlet–triplet energy gap: 2J) of photoinduced secondary CS states composed of flavin adenine dinucleotide radical anion (FAD−•) and radical cation WB(H)+• in the cryptochrome DASH from Xenopus laevis were clarified by time-resolved electron paramagnetic resonance. We found a time-dependent energetic disorder in 2J and was interpreted by a trap CS state capturing one reorientated water molecule at 120 K. Enhanced electron-tunneling by water-libration was revealed for the terminal charge-separation event at elevated temperature. This highlights importance of optimizing the electronic coupling for regulation of the anisotropic RP yield on the possible magnetic compass senses.
Similar content being viewed by others
Introduction
Various animals undergo migratory journeys guided by the Earth’s magnetic field1,2. Several proposals have been put forward on the mechanism of biological magnetoreception3,4,5,6; however, important aspects are still unclear7,8,9. The perhaps most likely mechanism by which migratory birds sense the magnetic field of the Earth involves the blue-light photoreceptor protein cryptochrome3,4,10, which was also supposed to be used in signaling in plants and in other animals8. Cryptochrome appears to be a quite versatile protein whose role varies depending on the signaling process and the respective organism, be it the entrainment of the circadian clock in vertebrates11, the regulation of stem elongation in plants, and so on12,13.
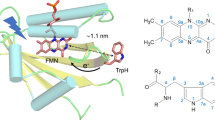
Most members of the cryptochrome protein family exhibit homology in three-dimensional fold, conservation of critical amino acids, and use flavin adenine dinucleotide (FAD) as redox-active cofactor8,14. Blue-light induced electron transfer (ET) leads to the generation of long-range charge-separation (CS) state14. Several studies highlighted the light-induced ET activity from the protein surface toward the FAD under the participation of redox-active tryptophan residues (Fig. 1)15,16,17. Upon photo-excitation, the FAD in its excited singlet state abstracts an electron from the nearby tryptophan WA(H) which is part of the so-called conserved “Trp-triad” of WA(H)···WB(H)···WC(H)18,19. Thus, a short-lived radical pair (RP) composed of the semiquinone anion radical, FAD−•, and a tryptophan cation radical, W(H)+•, is generated, which represents the primary CS state, i.e. FAD−•···WA(H)+•. Subsequently, quick stepwise sequential electron-hole transfers along the Trp triad take place until the terminal surface-exposed tryptophan (WC) forms a highly separated RP state FAD−•···WC(H)+•. This CS state may further be stabilized by deprotonation to a water molecule forming the RP of FAD−•···WC•7. Recently, a fourth tryptophan WD(H) was demonstrated to be oxidized, thus generating an even more separated RP state FAD−•···WD(H)+• as the terminal CS state in Drosophila melanogaster cryptochrome (DmCry) and in pigeon cryptochrome clCRY420,21,22.
X-ray structure of the animal-like cryptochrome of Chlamydomonas reinhardtii (PDB code:5ZM0) with the Trp triad comprising WA(H), WB(H) and WC(H)17.
In animal cryptochromes the stepwise charge separations proceed from the primary CS state of FAD−•···WA(H)+•, resulting in the terminal CS state15. It is essential that the electrostatic stabilizations of the intermediate CS states [primary CS state FAD−•···WA(H)+•···WB(H)···WC(H) and the secondary CS state FAD−•···WA(H)···WB(H)+•···WC(H)] are overcome for the ultimate oxidation of the terminal tryptophan to take place. Predictions from the theory have been put forward on how light-induced exergonic oxidization takes place at the terminal tryptophan residue through the Trp triad by stepwise ET16,23,24. As an example, it was predicted that the conformation of WB(H) changes after its oxidation, thus leading to a stabilization of the secondary CS state by the coordination of WB(H)+• to a threonine residue23,24. Water solvation is another origin of the exergonic oxidizations of WB(H) and WC(H) because of the higher water accessibility to residues located near the protein surface16. If a reorientation of one water molecule is preferential after the charge-separation, no conformation change in WB(H)+• is required. A recent molecular dynamics (MD) simulation study emphasized the role of forming a hydrogen-bond network involving a captured water molecule located between WB(H) and WC(H) in photoactivation by the stepwise CSs in a plant (6–4) photolyase25.
An advantageous point of utilizing the photoreceptor protein would be the strict controls of distances and orientations (i.e., electronic couplings) between the chromophore and residues for the terminal CS. Moreover, the water fluctuation can be proposed to be a key concept of generating the weak field-sensitive RP. Such solvent dynamics may be a promising perspective to control the electronic couplings applicable to energy conversions with sensing the Earth magnetic field. However, characterization of the electronic coupling has been a challenging task with tracking the time-courses of the intermediate geometries and of the solvation dynamics26. Thus, there is no experimental manifestation how the water dynamics can control the electronic coupling in the stepwise CS sequences for the field-sensitive RP7. Whereas conformational geometries were investigated on the terminal CS states using time-resolved electron paramagnetic resonance (TREPR)14,20,22,27, experimental evidence is still lacking on the molecular geometries, the electronic coupling and the electronic energy disorders by protein environment at the secondary CS state FAD−•···WB(H)+• and at the terminal CS state. One can thus raise the following fundamental questions on the cryptochrome: (1) Are conformation changes of FAD and/or WB(H) required after the secondary charge separation step to stabilize FAD−•···WA(H)···WB(H)+•···WC(H) with respect to the primary CS state? (2) Which role in magnetic compass sensing does the protein environment including specific water molecules play for the energetics and the electronic character in the CS states?
The TREPR method is particularly powerful for characterizing geometries of photoinduced radical pairs, as recently demonstrated for the primary CS state in plant photosystem II28, and for the quaternary CS states in cryptochromes from Chlamydomonas reinhardtii (ChlaCry) and Drosophila melanogaster (DmCry)20. Electron spin polarization (ESP) detected by TREPR as enhanced microwave absorption (A) and/or emission (E) from spin states of spin-correlated radical pairs (SCRP)14,29,30,31,32,33 is often sensitive to the direction of the external magnetic field (B0) with respect to the molecular frames of the RP constituents (specified by a set of coordinates Ω) because several interactions in the transient CS states are anisotropic. Therefore, one may obtain geometry parameters of transient states by analyzing the ESP pattern of SCRPs. Recently, the electron spin polarization imaging (ESPI) method to map the ESP for several B0-directions was introduced for a clear and direct visualization of geometries of transient CS states28,34. From a spectral analysis of TREPR data of the SCRP polarization obtained in thylakoid membranes, the anisotropy of the spin–spin dipolar coupling was mapped to the three-dimensional B0-direction to characterize the positions and the orientation of the primary CS state in the membrane28. Geometries and electronic singlet (S)-triplet (T) energy gaps (2J = ES–ET) were also investigated in CS states at bulk-heterojunction interfaces of organic solar cells (OCS) by analyses of the SCRP polarization in the donor (D)–acceptor (A) blend thin films of organic semiconductors34,35,36. 2J in the CS state has been treated to be a measure of the electronic coupling (V) for the long-range ET reaction by the configuration interactions from the charge-recombined singlet (1D···1A) and triplet (3D*···1A or 1D···3A*) electronic states through the V interactions37,38,39.
The objects of the present study are 1) clarifying molecular geometries of the secondary CS state, i.e. position of oxidized tryptophan and conformation of the reduced FAD in the secondary radical pair, and 2) understanding mechanism of the electron tunneling for generating the terminal CS state in relation to the signaling processes. For this we have measured and analyzed TREPR spectra of wild-type (WT) and mutant cryptochrome DASH from Xenopus laevis (XlCry-DASH). In the present study, time-dependent energetic disorders in 2J are found to be interpreted by a trapping of CS state capturing a reorientated water molecule at 120 K. Enhanced electron tunneling is also revealed by this fluctuating water for the terminal charge-separation at an elevated temperature.
Results
Work plan
We first characterize secondary CS state geometries and the electronic coupling using the light polarization (L) effect of the excitation laser on the TREPR spectrum with respect to the direction of the magnetic field (B0) for the WT protein at a cryogenic condition. This L effect, referred to as magnetophotoselection (MPS), was shown to be useful to determine the orientations of spin–spin dipolar interactions34,40,41,42. Secondary, we clarify that energetic disorder in the S–T gap is induced by sub-microsecond water dynamics causing a time-dependent heterogeneity in the exchange coupling of 2J in the CS state. Finally, we will discuss in details the electronic coupling matrix elements of the secondary and the ternary CS states by using 2J to clarify the role of the water fluctuation dynamics on the charge conduction through the cascade of the tryptophan residues.
TREPR spectra
We first observed the TREPR spectra at 240 K (Supplementary Figure 1) of the WT protein to confirm that the reported CS states were obtained as the E/A-polarized TREPR spectra assigned to the terminal RP comprising the reduced FAD and oxidized WC(H) (=W324 in XlCry-DASH)14,20,27. Panels a–c of Fig. 2 show TREPR spectra of WT XlCry-DASH obtained following photoexcitation with depolarized 450 nm-laser pulses at 120 K. The TREPR spectra are consistent with data reported previously43 and exhibit E/A/E/A patterns where E and A denote microwave emission and (enhanced) absorption, respectively. From the SCRP model specified in Fig. 2d, the four spin states (|1>, |2>, |3>, and |4>) are formed as a result of singlet(S)–triplet(T0) interaction in the presence of spin–spin-exchange (2J) and dipolar (d) coupling, thus leading to four EPR transitions29,30. The overall spectral widths of the E/A/E/A patterns depicted in Fig. 2 exceed 10 mT and are considerably larger than those of the RP spectra recorded at higher temperatures. We suggest that spin-spin coupling is larger at 120 K than at 240 K and contributes to spectral broadening, whereas at higher temperatures, the overall spectral width is mainly determined by anisotropic hyperfine interactions in the individual radicals of the SCRP. This implies that the primary or secondary CS state is generated, which leads to stronger spin–spin interactions due to the shorter distances between FAD and WA or WB (see Fig. 1) than the distance between FAD and WC.
a–c Delay time (td) dependence of the TREPR spectrum obtained by the depolarized 450 nm laser irradiation of WT XlCry-DASH at 120 K, showing E/A/E/A patterns. d The spin correlated radical pair (SCRP) levels (|1>, |2>, |3>, and |4>) via the superposition and subsequent decoherences by the interaction between the singlet (S) and T0 (|0>) states. The red arrows correspond to population relaxation determined by the rate of 1/T23 between the |2> and |3> levels contributed by J-modulation.
The overall width of the low-temperature TREPR spectra narrows with increasing delay time td after the laser flash, and also the E/A/E/A spectral shape changes, see Fig. 2a–c. This indicates that spin–spin exchange (2J) and/or the dipolar (d) coupling depend on td, thus suggesting that submicrosecond dynamic processes modulate the spin–spin interactions in the protein. Previously we presented a matrix formalism based on the Stochastic Liouville Equation (SLE)35,36,44,45 to analyze TREPR data of transient CS states, which can undergo singlet and triplet charge-recombination kinetics (kS and kT, respectively in Fig. 2d) and are subject to spin relaxations represented by (i) the relaxation time T23 between levels |2> and |3> (two arrows in Fig. 2d right) due to J-modulation7,46, and (ii) the spin-lattice relaxation time T1 determined by fluctuations in the anisotropic g-tensor, hyperfine-tensor and the dipolar coupling28,47. These relaxation effects may also alter the TREPR spectral shape and will be discussed below.
Magnetophotoselection effects on the TREPR spectrum of XlCry-DASH
From the SCRP model, it becomes evident that the two different contributions of d and 2J can be distinguished if MPS measurements are employed34,40. Fig. 3a shows a view of the principal axis (d) of the d-coupling between the radicals in the secondary CS state together with the B0 direction, and the transition dipole moment (M) for the S0–S1 optical absorption in FAD48. The peak-to-peak splitting (PPS = −4d + 2J) of the four EPR transitions (A and E in Fig. 2d) is contributed by the spin–spin dipolar coupling represented by d = D(cos2θD −1/3)/2 where D represents the dipolar coupling constant. This coupling thus depends on θD, i.e. the angle between B0 and d (see Fig. 3a) in the reference X-Y-Z coordinate49 system, while 2J is isotropic14,34. This isotopic J is valid in the present radical pairs that exhibit isotropic electronic coupling, as described below.
a Geometry setting of the secondary CS state with the transition dipole moment (M) lying in the FAD aromatic X-Y plane with δ = 65°. b The singlet precursor SCRP spectra computed for B0 // d (dashed line) and for B0 ⊥ d (solid line). c Magnetophotoselection (MPS) effects of the TREPR spectra for the delay times of td = 0.20, 0.45 and 0.60 μs at 120 K with B0 ⊥ L (dashed line) and B0 // L (solid line). d Computed EPR spectra of the SCRP for the B0 ⊥ L (dashed line) and for B0 // L (solid line) with applying D = −0.90 mT, θ = 58°, and ϕ = −65°. J = 1.45, 0.55 and 0.40 mT were applied for td = 0.20, 0.45, and 0.60 μs, respectively. T23 = 0.25 μs was utilized as the relaxation time constant between |2> and |3> by J-modulation, see Fig. 2d. e Mapping of the electron spin polarization (ESP) obtained by distributing the transverse magnetization (EPR intensities at B0 = 337.50 mT shown by blue arrow in b as the color map to the B0 space directions from the SCRP spectra at td = 0.45 μs, demonstrating that the d vector directs to WB(H) in the reference X-Y-Z coordinate49 system in a) of the protein.
Figure 3b shows singlet-precursor SCRP spectra for the B0 directions parallel (B0 // d) and perpendicular (B0 ⊥ d) to the inter-spin vector d in Fig. 3a calculated considering the SCRP level scheme shown in Fig. 2d. The broad E/A polarized spectrum (dashed line) for B0 // d is due to a large PPS (= 2.4 mT for θD = 0), when the Zeeman energy difference (ΔgβB0) determined by the g-factors (gFAD = 2.0034 and gW(H) = 2.0028)50,51 of the interacting two radicals is smaller than the PPS obtained for a dipolar coupling constant of D = −0.90 mT and an exchange interaction J = 0.60 mT. The E/A/E/A spin polarization for B0 ⊥ d in Fig. 3b is explained by a small PPS (= 0.6 mT for θD = π/2) by which the peak splitting is observed at each EPR line for gFAD and gW(H) resulting in two-antiphase doublets. The more intense inner A/E component in the red E/A/E/A pattern is due to |2>−|3> population relaxation induced by J-modulation, see Fig. 2d46.
To obtain this d anisotropy, we observed the MPS effects shown in Fig. 3c. The outer emissive and absorptive ESP signals of the E/A/E/A patterns are prominent in the spectra (dashed lines) recorded with the polarization of the laser (L) perpendicular (⊥) to B0. This indicates that the broad E/A contribution (dashed spectrum in Fig. 3b) is emphasized for B0 ⊥ L, thus implying that the interspin vector d directs far from the direction of M because B0 is situated to be perpendicular to M. Also, the spectra recorded with B0 // L in Fig. 3c are similar in shape to the E/A/E/A pattern (solid line for B0 ⊥ d) shown in Fig. 3b and are consistent with the above d tendency being perpendicular to M. These data are consistent with WB(H)+• being the radical species interacting with FAD•–, as shown in Fig. 3a. In Fig. 3d we show simulations of the experimental TREPR spectra using the SCRP model depicted in Fig. 2. The anisotropies of the g-matrices and the hyperfine tensors of the individual radicals, FAD−• and W(H)+•, together with the spin-relaxation and line broadening parameters compiled in Supplemental Table 1 were used for the spectral simulations28,34,40. See also Supplementary Figures 2-8 and Supplementary Tables 2 and 3 for details of the input parameters and their errors. The errors in the angles (θ and ϕ) and in the J were evaluated to be ±2 degrees and ±0.1 mT, respectively. The spectra in Fig. 3c were all reproduced with setting the d-direction to (θ, ϕ) = (58°, −65°) with δ = 65° (Supplementary Figure 2)48, as shown in Fig. 3d. The data show that at 120 K the secondary CS state is quickly generated within the instrumental response time and that the geometry of the CS state is independent of td. From the spectral simulations including the experiments with depolarized light (red lines in Fig. 2)34, one can distribute the ESP intensities of the transverse magnetization to all the field directions at a specified B0 strength (= 337.5 mT) to obtain the ESPI map shown in Fig. 3e28. This visualization clearly shows the orientation of the interspin vector of d between FAD−• and WB(H)+• with respect to the M direction in the X-Y reference axes in FAD. The dark red region in the map reflects the strong anisotropy (AZZ) of the nitrogen hyperfine interaction (Supplemental Table 3) in FAD−•, further supporting the d vector directing from the FAD−• aromatic plane to WB(H)+•. From D = −0.90 mT, which reproduced the MPS results in Fig. 3c very well, the separation distance between the spins is estimated to rCC = 1.45 (±0.08) nm using the point-dipole approximation. This distance is in good agreement with the center-to-center distance of 1.40 nm between flavin and WB(H), see Fig. 1. Furthermore, the following conclusions are derived from the MPS analysis and the ESPI mapping:(i) The FAD−• conformation in Fig. 3a remains unchanged with respect to the fully oxidized flavin molecule photoexcited along with the M direction in the protein, and (ii) the position of WB(H)+• is (θ, ϕ) = (58°, −65°), as in the x-ray crystal structure of Fig. 3a. Because a water molecule is located next to WB(H) (see Fig. 1), deprotonation could occur to generate the RP FAD−•···WB• with H3O+. This possibility is however excluded at 120 K because a large reorganization barrier of 1 eV is expected along the deprotonation reaction coordinate52. The spin density distributions predicted by the hyperfine couplings in WB(H)+• are also coincident with previous report41,53 as detailed in Supplemental Table 2. We thus conclude that the molecular geometries of FAD and WB(H) are both preserved after stepwise charge separations at 120 K.
Modeling time-dependent disorder in the S-T Gap
In the present line-shape analysis (Supplemental Table 1), heterogeneities in the 3d and d − 2J energies are determined by T2d* and T2J*, respectively44, and contribute to variation in the T0 − T± and S − T± gaps (solid and dotted arrows34,44 in Fig. 2d left), respectively. Thus, 1/(2πT2d*) and 1/(2πT2J*) are relevant to the T0 − T± and S − T± variations, respectively, for the EPR transitions in Fig. 2d right. The td dependence of the entire spectral line shape in Fig. 2 and in Fig. 3c was explained by a decrease in the 2J together with an increase in T2J* as td proceeds, resulting in the narrow E/A/E/A line at 0.6 μs. As a result, (J, T2J*) = (1.45 mT, 3 ns), (0.55 mT, 15 ns), and (0.40 mT, 20 ns) were obtained at td = 0.20, 0.45 and 0.60 μs, respectively, to fit the data as shown in Fig. 3d.
To examine whether the above EPR shape originating from the width represents the heterogeneity of the S–T gap or not, we plotted the EPR line-shape of the antiphase pattern, which is one E/A part of the E/A/E/A antiphase doublets of the SCRP spectrum29,30. This is simply confirmed by summing two of the Lorentz functions (Supplementary Equation (1)) in Supplementary Figure 9 assuming D = 0 for the |1>−|3> and |2>−|4> transitions. Because PPS of this E/A polarization line (Supplementary Figure 9) is determined by the single input value of 2J29,30, and because the spectral width reflects 1/(2πT2J*) as an uncertainty in J, the high-field side from the center of the E/A line represents a distribution function of the S-T gap. From this, the distribution functions of 2J were derived using Supplementary Equation (2) in Supplementary Figure 9, as shown in Fig. 4a for the above (J, T2J*) parameters. Notably, the width in the 2J distribution was identical with the 1/(πT2J*) value (= 4 mT for T2J* = 3 ns) as shown by the solid arrows in Fig. 4a, showing that the 2J distributions is dependent of td. See Supplementary Figures 10–12 for more details on the validity of the present treatment of the 2J distributions using the lifetime broadening effect.
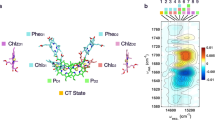
a Time (td) dependent distributions of the S-T gap (2J) of the secondary CS state derived from the higher-field curve (Supplemental Figure 9) of the E/A antiphase EPR line-shape for 1) td = 0.20, 2) 0.45, and 3) 0.60 μs at 120 K from the right to left, respectively. b Potential energy surfaces of the excited singlet state (1FAD*), the triplet state (3FAD*), the secondary CS state (FAD−•···WB(H)+•) and the ground state (S0). The CS state energy distributions are caused by solvent dynamics, as represented by the Gaussian functions at 1) td = 0.20, 2) 0.45, and 3) 0.60 μs shown by the dotted lines. The computed S–T gap (= 2JCT) from the configuration interaction model of Eq. (1) is plotted by the solid red line. c The population distributions (dotted lines in b) are plotted as the function of the 2JCT (red line in b) for td = 0.20, 0.45, and 0.60 μs from the right to left, respectively.
From the MPS analysis the geometry of the CS state was revealed to be td-independent. On the other hand, 2J and its distribution both vary with td. Thus, the decay and the disorder of 2J are not likely to originate from geometry changes of the radicals of the CS state but from td-dependent disorder in the solvent coordinate (X) of the CS state, i.e., from solvation dynamics. To ascertain this notion, four potential energy curves (ES1, ET1, ECS, and ES0) were drawn for the excited states of the FAD, 1FAD*, and 3FAD*, the secondary CS state (FAD−•···WB(H)+•), and the ground state (S0), respectively (Fig. 4b). These energies were estimated by using reported excited-state energies48,54, electrochemical potentials (Ered = −0.38 V for FAD/FAD−• and Eox = 1.15 V for W(H)/W(H)+• vs. NHE)52,55 and the reorganization energy of λ = 0.41 eV applicable to the protein environment56.
From the configuration interaction model for the long-range CS state systems37,38, the S-T energy gap of 2JCT is induced by electronic-coupling (V) perturbations from the CR configurations, as expressed by
where S1 = 1FAD* and T1= 3FAD* in Fig. 4b. From Eq. (1), 2JCT(X) was calculated as shown by the bold line in the bottom of Fig. 4b with setting |VS1| = 6.3 cm−1, |VT1| = 5.6 cm−1 and |VS0| = 2.0 cm−1 for the CRs to 1FAD*, 3FAD* and S0, respectively. These couplings are rationalized by the McConnell superexchange model57 through WA(H), excluding anisotropy in J as detailed in Supplementary Note 158.
|VFAD*,secondary| = (|VS1| + |VT1|)/2 = 6.0 cm−1 is thus evaluated as the electronic coupling leading to the excited FAD* from the secondary CS state. It is anticipated that the S–T gap significantly decreases as X proceeds, as shown by the red arrow in Fig. 4b because of the increase in the gap between \(E_{{{{{{{{\mathrm{T}}}}}}}}1}\left( X \right)\) and \(E_{{{{{{{{\mathrm{CS}}}}}}}}}\left( X \right)\) in Eq. (1). This is relevant for the significant decrease in the peak position of 2J (Fig. 4a) by td. From these peak values of 2J (= 2.9 mT, 1.1 mT, and 0.8 mT) in Fig. 4a, one can thus estimate the X positions (Xp = 0.35, 0.67 and 0.80, respectively) that give the peak of the 2J-distribution in X, as shown by the vertical dashed arrows for 2J = 2.9 mT (at td = 0.20 μs) in Fig. 4b. Accordingly, the distribution function of the CS states are depicted by the normalized gaussian function of \(\sqrt {\frac{\lambda }{{\pi k_BT}}} \exp [ { - \lambda (X - X_p)^2/\left( {k_BT} \right)} ]\), as the dotted curves in Fig. 4b. From these distributions and 2JCT(X) of the red line in Fig. 4b, one can evaluate the distribution of the 2JCT values, as shown in Fig. 4c. The good agreements between Figs. 4a, c demonstrate that the solvation dynamics in Fig. 4b play a role for the time-dependent disorder in 2J. More details on the connection between the electron transfer mechanism and the present solvation dynamics59 are described in Supplemental Figure 11.
Protein solvation dynamics in the CS state
To evaluate the solvation time constant, the (1 − Xp) values in Fig. 4b were plotted against td, as shown by the semi-log plot of Fig. 5a. The plot obeys a single-exponential decay (solid line in Fig. 5a) with a lifetime of τ = 0.34 μs in Fig. 4b. Notably, this relaxation time is coincident with T23 ≈ 0.25 μs utilized as the J-modulation induced spin-relaxation60 between |2> and |3> in Supplemental Table 1. These findings suggest that reorientations of polar groups in amino-acid residues and/or of water molecules nearby FAD−• and WB(H)+• (W377+• in XlCry-DASH) contribute to relaxation. In particular, a local structural rearrangement close to WB(H)+• is the most plausible cause after the charge-shift from WA(H)+• to WB(H) during stepwise CS. Such slow protein dynamics at cryogenic temperatures were previously reported for the photosynthetic reaction center from Rps. sulfoviridis and were thought to arise from the reorientation of individual water molecules56. Furthermore, one water molecule (Wat830 in Fig. 1) is found between WB(H) and WC(H), forming a hydrogen bond network near the protein surface. Hosokawa et al25. suggested that the long-range hydrogen bond network is extended by this captured water along the Trp triad and may thus play a role for photoactivation in plant photolyase. This water-binding site (Supplemental Figure 13) was also found in a pigeon cryptochrome of Columbia livia61.
a Solvation dynamics obtained by the decay of 1 − Xp in WT XlCry-DASH (•) and in W342F (▴) at 120 K. b X-ray structure-based water conformation confined by WB(H), WC(H), and −C=O groups of the backbone at R310 and the N317 residue of Synechocystis sp. PCC6803 cryptochrome DASH. c Water orientation change by the rehydration (red dotted line) to the cationic charge of WB(H)+• after the secondary CS. The weakened hydrogen bonding between the water and WC(H) hinders the ternary CS by this trapping, indicating the importance of thermal water fluctuations for the terminal CS resulting in WC(H)+• at physiological condition.
Figure 5b shows the anticipated molecular conformation of the bound water molecule from the reported x-ray structure of Synechocystis sp. PCC6803 cryptochrome DASH (PDB code: 1NP7)18. In addition to WB(H) and WC(H), a −C=O group of the backbone at L318 participates in water binding (see Fig. 1), thus resulting in hydrogen bonding of −C=O···HOH. The −C=O groups were also identified from R310 and N317 in the cryptochrome DASH of Synechocystis sp. PCC6803: they face toward WB(H) and WC(H) (see Fig. 5b) although the captured water molecule was not detected by x-ray crystallography. This indicates that one water molecule is likely to be captured in this specific cavity nearby the protein surface in XlCry-DASH. From the crystal structure shown in Fig. 117, the hydrogen bond distance between WC(H) and the oxygen of Wat830 is r(NC···O) = 3.01 Å; a similar value is found for the respective distance of WB(H): r(NB···O) = 3.00 Å. This suggests that this bonding network could be utilized for the electron-tunneling routes62 at the ternary CS to oxidize WC(H), which will be detailed below.
After the secondary CS at the cryogenic temperature, it is expected that the distance r(HB···O) becomes shorter on tightening the HB···O bond due to the positive charge of WB(H)+•, as shown by the red dotted line in Fig. 5c. This rehydration thus weakens the HC···O interaction to hinder the electronic coupling for the terminal CS, which is consistent with the present assignments as the secondary CS state from the SCRP analysis of the TREPR spectra, see Fig. 3. With reforming the hydrogen bonds, the water molecule is expected to be slowly reoriented by the turn of the O-H group (Fig. 5c). This contributes to the td-dependent disorder in 2J by protein reorganization (see Fig. 4) at T = 120 K. The slow solvent dielectric relaxation (τ = 0.34 μs in Figs. 4b and 5c) is not surprising in frozen environments. It is well known that microsecond dielectric relaxations of glass-forming solvents, such as alcohols and 2-methyltetrahydrofuran, determine dynamic spectral shifts and electron transfer (ET) kinetics around 100 K63,64,65. λ = 0.41 eV, which is much smaller than in bulk water solution66 (see Fig. 4b), is also well compatible with shifts (ca. 0.4 eV) of the vertical photo-detachment energies in fluorenone anion radicals by forming the 1:1 and 1:2 clusters with methanol in the gas phase67, thus suggesting that reorientation stabilization associated with a couple of the hydrogen bonds (Fig. 5b) largely contributes to the potential surface in the CS state at this cryogenic condition. This reorganization energy excludes light-induced proton-coupled ET generating FAD−•···WB• with H3O+, because a very large reorganization energy of 1 eV is required on the protonation/deprotonation reaction coordinate in Fig. 4b52. The dielectric relaxation time (τ = 0.34 μs) is much shorter than the solvation time (>10−6 s) in bulk frozen media at 120 K65. The present rapid dielectric response thus indicates that the local water molecule participating in the hydrogen bond network25 is involved in solvation dynamics, as opposed to the condensed phase solvent packing situations in the frozen solution. Slightly larger isotropic proton hyperfine couplings in WB(H)+• were utilized in Supplemental Table 2 to reproduce the line shapes in Fig. 2 than the couplings for the terminal CS state in Supplemental Figure 1. This might include effects of the superhyperfine couplings62 of the reorienting water to WB(H)+• in Fig. 5c.
To examine more details of the solvation dynamics, we observed the TREPR spectra of a mutant of XlCry-DASH, W324F at 120 K, see Supplemental Figure 7a–c. While the E/A/E/A spin polarization pattern of the mutant is very similar to that of the WT, the inner A/E polarization component becomes quickly stronger than the outer E/A component. This is rationalized by the quick |2>−|3> relaxation by the J-modulation in Fig. 2d and was consistent with the quick decrease in the J coupling (Supplemental Table 4). From the fitting lines in a)-c) of Supplemental Figure 7 and the time dependence of 2J, we also estimated 1 − Xp values which decay is shown in Fig. 5a to obtain the solvation time of 0.16 μs.
Discussion
Water reorientation as the origin of the time-dependent exchange coupling
The shorter solvation time (0.16 μs from the red dotted line in Fig. 5a) in the mutant as compared to the WT is a strong indication that the local water solvation dynamics dominates the dielectric response and is rationalized by the absence of a hydrogen bond between the water and phenylalanine (see Supplemental Figure 7d). This is because the water molecule is anticipated to rotate more freely in the cavity area, when the hydrophobic phenylalanine residue does not participate in the hydrogen-bond network. In turn, the slower solvation dynamics is considered for the WT to be caused by the reorganization of the hydrogen bond network around WB(H)+• (Fig. 5b). Notably, the secondary CS state geometries were revealed to be very consistent with the positions of FAD and WB(H) of the x-ray structures. This is most consistent with the water reorientation mechanism, as detailed below. As an example, if the C=O group of R301 in Fig. 5 directly ligated to WB(H)+• after the charge-separation at 120 K, the position of WB(H)+• is required to be changed in the X-Y-Z coordinate system of FAD and must have altered the magnetophotoselection results. In previous MD simulation studies on the ET reactions of cryptochrome and photolyase, conformational changes of the residues nearby WB(H)+• and FAD−• were predicted during the charge-separation23. On the secondary CS state, large degrees of displacements, greater than a few Å, were predicted both for WB(H) and for threonine residues at room temperature during protein dynamics assisted by the thermal energy for the exergonic WB(H) oxidation by ligating the polar groups to WB(H)+•. This mechanism is however excluded at cryogenic temperatures, such as the ones considered in this study; thermal activations of protein vibrations were shown to be highly restricted below 150 K68. The proposal of water reorientation mechanism with minimal protein displacements (Fig. 5c) is rather reliable scenario for explaining of both (i) the dielectric stabilization dynamics of the secondary CS state and (ii) the blocking of the terminal CS to oxidize WC(H) at 120 K. The theoretical predictions of the changes in the molecular positions and conformations of the residues and FAD were reasonable when the photoinduced radical species becomes newly bound to one of the polar groups of another residue because the whole protein molecule possess the self-organized 3D structure via polypeptide chains. On the other hand, the d-direction of (θ, ϕ) = (58°, −65°) with δ = 65° in Fig. 3a is concluded to be time-independent and is consistent with the x-ray structures. This is interpreted by the single water conformation change bound to WB(H)+• to stabilize the radical pair causing the time-dependent distributions in the S-T gaps as shown in Fig. 4b, although the other environmental effects would participate. FAD is known to be located at the hydrophobic region inside the protein. Thus, the water reorientation around FAD−• is not plausible. Possibilities of glycerol binding effect and its reorientation dynamics are also excluded, as detailed in Supplemental Note 2 with Supplemental Figures 13 and 14.
Fluctuating captured water promotes electron tunneling in magnetophotoreception
At 240 K, the SCRP spectra of the terminal CS state, FAD−•···WC(H)+•, were well reproduced (red lines in Supplemental Figure 1) by setting a td-independent J parameter with J = 22 μT for td > 0.1 μs (see METHODS and Supplemental Table 5) using the x-ray conformations of FAD···WC(H) shown in Fig. 3a with the inter-spin distance of rCC = 1.91 ± 0.08 nm, as detailed in Supplemental Figure 2. Although this rCC is well compatible with values obtained from EPR studies of the FAD−•···WC(H)+• distances14,20,21,22, the present geometries of the secondary (Fig. 3a) and ternary CS states are in conflict with ultrafast transient absorption anisotropies by which the holes in the sequential CS states were suggested to be delocalized in the Trp-triad15. The td-independent J at 240 K reflects a rapid dielectric response to result in Xp = 1 (see Fig. 4b)69. Thus, the picosecond vibrational cooling via fast water reorientations induces the localized holes at the sub-microsecond domains, while the CS states could maintain the hole delocalization at the picoseconds time regime15 because of the involvement of the vibrationally hot CS. Therefore, a quick response via the hot CS in Supplemental Figure 11 would play a role on the secondary CS, as has been discussed on the initial charge-generations at the bulk-heterojunction interfaces of the OCS26,34,70.
From X = 1 in Eq. (1), |VS1,teminal| = 1.6 cm−1 and |VT1,teminal| = 1.5 cm−1 were obtained at 240 K as the electronic couplings of the terminal CS state to FAD* using J = 22 μT as detailed in Supplemental Table 5. As a result, the electronic coupling (|VFAD*, terminal| = 1.5 cm−1 to FAD*) in the terminal CS state is attenuated from |VFAD*| = 6.0 cm−1 of the secondary CS state (120 K). From the superexchange model57, |VFAD*, terminal| is expressed45 as follows:
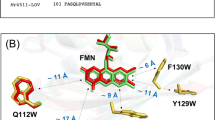
where |VHH|, |VHHAB|, and |VHHBC| represent transfer integrals28,41 between the highest occupied molecular orbitals (HOMOs) as shown in Fig. 6 and in Supplemental Figure 15. ΔEHH (≈ λ = 0.41 eV) is the vertical energy gap for the hole-transfer between WB(H) and WC(H)+• and is largely caused by the water solvation (Fig. 4b) in the present system. |VHH| ≈ |VHHAB| ≈ |VHHBC| is assumed41,45 at higher temperature because corresponding edge-to-edge separations (<0.4 nm in the dashed lines of Fig. 6) are largely common. From Eq. (2), |VHHBC| ≈ 250 cm−1 is thus estimated at 240 K on the hole-transfer between WB(H) and WC(H) (see Fig. 6 and Table 1). This value would be too large for the transfer integral between WB(H) and WC(H) because |VHHAB| = 140 cm−1 in the secondary CS state (Supplemental Figure 15) was consistent with the reported couplings (≈ 100 cm−1)41 for the contact edge-to-edge separations58. The electronic coupling was previously demonstrated to be mediated through an intervening bound water in a triplet-triplet energy transfer system at the photoprotective site of the peridinin–chlorophyll a–protein from Amphidinium carterae62. Thus, |VHHBC| ≈ 250 cm−1 is characteristic of the tunneling via the water (see Fig. 6) particularly at higher temperatures.
Estimated based upon the x-ray structure of Synechocystis sp. PCC6803 cryptochrome DASH. Black dashed lines denote nearest-neighbor edge-to-edge separations. |VHH| ≈ |VHHAB| ≈ 140 cm−1 was estimated in FAD−•···WB(H)+• at 120 K (Table 1). Bold dotted lines are the hydrogen bond separations (R = 0.3 nm) by the water for mediating the transfer integral (|VHHBC| ≈ 250 cm−1) at 240 K. Structural rearrangements are anticipated in the blue backbones due to the cationic charge generation at WC(H)+•.
The attenuation degree (ε) of the electronic coupling by elongation of the distance between the secondary and terminal CS states is represented as, ε = |VFAD*,terminal|/|VFAD*,secondary|. ε = |VHHBC|/ΔEHH = |VHHBC|/λ is derived with substituting |VFAD*,secondary| = |VHHAB||VHH|/ΔEHH (Supplemental Figure 16)45 to Eq. (2), and thus is evaluated to be ε = 0.08 from |VHHBC| ≈ 250 cm−1 at 240 K. This perfectly agrees with the Pathways model71 presented by Beratan: the attenuation in |VFAD*| is ε = 0.07 via the two hydrogen-bond steps (two dotted lines in Fig. 6) as a product (εS1εS2) of the per-unit penalties (εS)72 with εSi = (0.36) × exp[−11 (Ri − 0.28 nm)] by the jumps in the lengths of Ri = 0.30 and 0.31 nm between N···O atoms in Fig. 1 (Table 1). Because the Pathways model is widely applicable to account for the ET rate constants particularly in systems where tunneling matrix elements are sensitive to the collective thermal equilibrium motions in proteins72, |VHHBC| ≈ 250 cm−1 reflects amplifications of the coupling due to the thermally assisted non-Condon effect in the protein73. This is relevant to the entropy mechanism by which the density of states is enhanced by the electron-phonon coupling for the exothermic carrier dissociation in the OCS26,44,74. The trapping feature of the secondary CS state is thus relevant to the reported bound electron-hole pairs with separation distances around 2 nm at the D:A interfaces in the OCS, which was explained by the restraint of the electron-phonon coupling at T = 77 K.
The thermally amplified VHHBC in Fig. 6 strongly supports the slow water-trapping scenario on the secondary CS state in Fig. 5c; insufficient water-fluctuations and the resultant rehydration should reduce the |VHHBC| coupling and block the terminal CS generations at 120 K even if the hot CS-state is initially generated15, leading to the submicrosecond reorientation relaxations. The amplifying phonon modes associated with the bound waters were recently clarified using terahertz spectroscopy on hydrated nylon polymer;75 the vibration frequencies of the bound-water motions were found to be higher than 4 THz, which corresponds to an energy that is not thermally activated at 120 K with kBT = 2.5 THz but can be accessible at 240 K (5 THz). Kobori et al.39 reported that the solvent-solvent effective electronic coupling (|VSS| ≈ 850 cm−1) was highly amplified by solvent fluctuations at a mean solvent-solvent distance of 0.57 nm in the condensed media from analyses of the chemically induced dynamic electron polarization by radical ion pairs in the liquid solutions. The present enhanced |VHHBC| interaction at 240 K is compatible with this report. Moreover, |VHHBC| which is weaker than |VSS| ≈ 850 cm−1 is explained by suppression of the large-scale collective fluctuations at the local protein cavity where significant water-water fluctuations around the protein hydration layer are inhibited.
Finally, a repulsion between WC(H)+• and R479+ is expected to occur at the ternary CS in Fig. 6 because the arginine residue is deprotonated due to pKa = 12.5. This should cause breaking of the cation-π interaction between R479+ and WC(H) (dashed line in Fig. 6) to release and unfold the C-terminal chain together with subtle rearrangement of the backbone at R310+ and N317 by the rehydration to WC(H)+•. Thereby the captured single water would effectively trigger the light-induced signaling processes at least in Synechocystis sp. PCC6803 cryptochrome DASH76, as well as the regulations in the electron tunneling at the physiological condition, as discussed above.
Conclusion
We have experimentally characterized the molecular geometries, solvation dynamics and electronic couplings of the secondary and ternary photoinduced charge-separated (CS) states in WT XlCry-DASH at T = 120 K and 240 K, respectively, using the TREPR method. It is concluded that the nuclear displacements by the stepwise charge-separations are minor both in the reduced and oxidized components from the conformations of (θ, ϕ) = (58°, −65°) with δ = 65° at 120 K. Based upon this, the small degree of the attenuation in the electronic coupling (Table 1) is explained by the motions of the captured single water molecule (see Fig. 6) playing a significant role for mediating the long-range electron-tunneling at 240 K. In particular, thermally assisted water fluctuations at terahertz frequencies77 are a key factor to prohibit the slow solvation at the secondary CS (Fig. 5c) and to facilitate subsequent charge separations. The present thermal-equilibrium motional assistance coincides with previous predictions by MD simulations16 that described the picosecond fluctuations in the transfer integral (VHHBC) between WB(H)+• and WC(H) frequently amplified to VHHBC > 50 cm−1 during the ternary charge-separation event. The solvent-mediated effective tunneling of |VHHBC| ≈ 250 cm−1 in the protein cavity is however weaker than the thermally activated inter-solvent coupling (|VSS| ≈ 850 cm−1) in the liquid phase. This optimized tunneling matrix element is crucial for regulations of the anisotropic CR of the terminal CS states utilized for the possible magnetic compass senses with the signaling processes6,8,11, because the terminal RP yield can be determined by the competition7 between the singlet recombination via |VHHBC| and the anisotropy in the singlet-triplet spin conversion around the strength of the Earth magnetic field.
Methods
Sample Preparations
The gene encoding XlCry-DASH, which was cloned into pGEX4T-2 vector78, was inserted between the NdeI and XhoI sites of a modified vector, pET-28a expression vector (Novagen) whose kanamycin-resistant cassette was replaced with ampicillin-resistant cassette from pET-21a vector (Novagen). A mutant (W324F) of XlCry-DASH was constructed by PCR using the QuikChange site-directed mutagenesis method (Agilent Technologies). The WT and mutant XlCry-DASH proteins were expressed in E. coli BL21(DE3) as a fusion protein with His6-tag at the N-terminus. Protein expression and purification condition was carried out in accordance with the method of Xl (6–4) photolyase79. After purification, buffer exchange was carried out by dilution with 0.3 M NaCl, 0.1 M Tris·HCl, pH 8.0, 30% (v/v) glycerol, and concentration by ultracentrifugation devices (Amicon Ultra-15 and -0.5 mL, Merck) for a few times. Because our XlCry-DASH contained fully reduced and neutral semiquinoid forms, potassium ferricyanide was added at a final concentration of 5 mM in order to oxidize the samples to fully oxidized form80. It took about 5–7 days at 4 °C in the dark to make the samples fully oxidized form. To remove potassium ferricyanide, dilution with the same buffer and concentration by ultracentrifugation was carried out for a few times.
The sample solutions (0.3 M NaCl, 0.1 M Tris·HCl, pH 8.0, 30% (v/v) glycerol) were deoxygenated by the freeze-pump-thaw cycles and were transferred to sample tubes with diameters of 5.0 and 0.6 mm for the measurements at 120 and at 240 K, respectively. These tubes were sealed using a torch for the TREPR measurements. Nitrogen gas was introduced before the sealing of the 0.6 mm-diameter tube.
To check a possible effect of impurity by potassium ferricyanide to oxidize the protein, chromophore-removed XlCry-DASH (apo-XlCry-DASH) was subjected to ferricyanide treatment. The chromophores were removed by the dialysis against 2 M KBr, 100 mM KCl, 10 mM 2-mercaptoethanol, 1 mM EDTA, and 50 mM Tris-HCl, pH 4.0 at 4 °C for 6 days81. The soluble protein was collected and the buffer was exchanged to 0.3 M NaCl, 0.1 M Tris-HCl, pH 8.0, 30% (v/v) glycerol by Amicon devices. The protein concentration was measured using Bio-Rad Protein Assay Kit, based on the Bradford protein assay, with BSA solution as a standard. The treatment and removal of potassium ferricyanide of the apo-XlCry-DASH was carried out in the same way as the treatment of the chromophore-bound XlCry-DASH.
The molar absorption coefficient of the chromophore removed sample was estimated from the protein concentration, the molecular mass of the calculated His6-tagged XlCry-DASH (62.8 kDa), and the absorption spectrum, which was estimated to be ε257nm = 3.61×105. UV-vis spectra of apo-XlCry-DASH with and without ferricyanide treatment as well as chromophore-bound XlCry-DASH were shown in Supplementary Figure 17. Oxidized forms of amino acid species were evaluated to be minor by the optical absorption bands from 300 to 500 nm for tryptophan82 and for tyrosine83. The positions of the tryptophan and tyrosine side chains that could be oxidized were estimated based on the crystal structure of Synechocystis sp. PCC6803 cryptochrome DASH18 (Supplementary Figure 18).
Time-resolved EPR measurements
The X–band TREPR measurements were performed using a Bruker EMX Plus system in which a modified wide-band preamplifier was equipped in the microwave bridge. The field modulation was not employed. Light excitations were performed by Continuum optical parametric oscillators (OPO) systems (Surelite OPO Plus) pumped with a third harmonics (355 nm) of a Nd:YAG laser (Continuum, Surelite I-10, 5 ns). A laser de-polarizer (SIGMA KOKI, DEQ 1N) was placed between the laser exit and the microwave cavity for the depolarized TREPR data. For the MPS, a polarizer (SIGMA KOKI, WPQW-VIS-2M) was used to rotate the laser polarization direction by 90 degrees. Transient EPR signals were averaged by a Tektronix DPO3054 500 MHz digital phosphor oscilloscope at 201 different external magnetic field positions34. Temperature was controlled by a cryostat system (Oxford, ESR900) by using liquid nitrogen as the cryogen84.
Numerical simulations of the TREPR spectra
Numerical calculations of the EPR spectra were performed using MATLAB (The MathWorks) codes. The computation methods to obtain the delay time (td) dependence of the TREPR spectrum28,36,45 and the time profiles35 of the transverse magnetizations were reported previously for the transient CS states. We set a precursor CS state in which density matrix elements of coherence terms (ρ0S and ρS0) in an S-T0 basis system developed from 0 ns to the time of td in the presence of a strong exchange coupling (Jpre = 3 mT) for the secondary CS state34. Then, such coherence terms together with the populations (ρSS and ρ00) were transferred to the corresponding density matrix elements of the SCRPs to generate the resultant density matrix elements85. This will produce an overlapping net ‘E’ polarization in the FAD−• resonance field and a net ‘A’ in the higher field W(H)+•, which is originating from the chemically induced dynamic electron polarization (CIDEP) from the precursor radical pairs85,86,87. For the MPS analysis40, the ESP signals (ESP0para and ESP0perp) for B0 // L and B0 ⊥ L, were set to be proportional to the squares of direction cosines and to the halves of the squares of the sine components, respectively, between the B0 and M vectors (Fig. 3a). As for the terminal CS state (Supplemental Figure 2) obtained at 240 K, Jpre = 0.1 mT was considered. The ρ0S and ρS0 terms were set to be transferred to the corresponding density matrix elements of the SCRPs in a 1 ns time constant because of the quick solvent relaxation in Fig. 4b. In this case, the CIDEP effect was very minor in the ESP.
Data availability
The data associated with the reported findings are available in the manuscript or the supplementary information. Other related data are available from the corresponding authors upon request.
Code availability
The MATLAB codes are available from Yasuhiro Kobori upon request.
References
Lohmann, K. J. Magnetic orientation by hatchling loggerhead sea turtles (Caretta caretta). J. Exp. Biol. 155, 37–49 (1991).
Collett, T. S. & Baron, J. Biological compasses and the coordinate frame of landmark memories in honeybees. Nature 368, 137–140 (1994).
Ritz, T., Adem, S. & Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718 (2000).
Kirschvink, J. L., Walker, M. M., Chang, S. B., Dizon, A. E. & Peterson, K. A. Chains of single-domain magnetite particles in Chinook Salmon. Oncorhynchus-Tshawytscha. J. Comp. Physiol. A 157, 375–381 (1985).
Mouritsen, H. & Hore, P. J. The magnetic retina: light-dependent and trigeminal magnetoreception in migratory birds. Curr. Opin. Neurobiol. 22, 343–352 (2012).
Hore, P. J. & Mouritsen, H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 45, 299–344 (2016).
Maeda, K. et al. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl. Acad. Sci. U. S. A. 109, 4774–4779 (2012).
Dodson, C. A., Hore, P. J. & Wallace, M. I. A radical sense of direction: signalling and mechanism in cryptochrome magnetoreception. Trends Biochem. Sci. 38, 435–446 (2013).
Babcock, N. S. & Kattnig, D. R. Electron–electron dipolar interaction poses a challenge to the radical pair mechanism of magnetoreception. J. Phys. Chem. Lett. 11, 2414–2421 (2020).
Lohmann, K. J. Magnetic-field perception. Nature 464, 1140–1142 (2010).
Berntsson, O. et al. Photoactivation of Drosophila melanogaster cryptochrome through sequential conformational transitions. Sci. Adv. 5, eaaw1531 (2019).
Sang, Y. et al. N-terminal domain-mediated homodimerization is required for photoreceptor activity of Arabidopsis C ryptochrome 1. Plant Cell 17, 1569–1584 (2005).
Pokorny, R. et al. Recognition and repair of UV lesions in loop structures of duplex DNA by DASH-type cryptochrome. Proc. Natl. Acad. Sci. U. S. A. 105, 21023 (2008).
Weber, S. et al. Origin of light-induced spin-correlated radical pairs in cryptochrome. J. Phys. Chem. B 114, 14745–14754 (2010).
Lacombat, F. et al. Ultrafast oxidation of a tyrosine by proton-coupled electron transfer promotes light activation of an animal-like cryptochrome. J. Am. Chem. Soc. 141, 13394–13409 (2019).
Lüdemann, G., Solov’yov, I. A., Kubar, T. & Elstner, M. Solvent driving force ensures Fast Formation of a Persistent and Well-Separated Radical Pair in Plant Cryptochrome. J. Am. Chem. Soc. 137, 1147–1156 (2015).
Franz, S. et al. Structure of the bifunctional cryptochrome aCRY from Chlamydomonas reinhardtii. Nucleic Acids Res. 46, 8010–8022 (2018).
Brudler, R. et al. Identification of a new cryptochrome class: structure, function, and evolution. Mol. Cell 11, 59–67 (2003).
Park, H. W., Kim, S. T., Sancar, A. & Deisenhofer, J. Crystal-structure of DNA photolyase from Escherichia-Coli. Science 268, 1866–1872 (1995).
Nohr, D. et al. Extended electron-transfer in animal cryptochromes mediated by a tetrad of aromatic amino acids. Biophys. J. 111, 301–311 (2016).
Nohr, D. et al. Determination of radical-radical distances in light-active proteins and their implication for biological magnetoreception. Angew. Chem. -Int. Ed. 56, 8550–8554 (2017).
Hochstoeger, T. et al. The biophysical, molecular, and anatomical landscape of pigeon CRY4: A candidate light-based quantal magnetosensor. Sci. Adv. 6, eabb9110 (2020).
Solov’yov, I. A., Domratcheva, T. & Schulten, K. Separation of photo-induced radical pair in cryptochrome to a functionally critical distance. Sci. Rep.-Uk 4, 3845 (2015).
Solov’yov, I. A., Domratcheva, T., Moughal Shahi, A. R. & Schulten, K. Decrypting cryptochrome: revealing the molecular identity of the photoactivation reaction. J. Am. Chem. Soc. 134, 18046–18052 (2012).
Hosokawa, Y., Sato, R., Iwai, S. & Yamamoto, J. Implications of a water molecule for photoactivation of plant (6–4) photolyase. J. Phys. Chem. B 123, 5059–5068 (2019).
Imahori, H., Kobori, Y. & Kaji, H. Manipulation of charge-transfer states by molecular design: perspective from “Dynamic Exciton”. Acc. Mat. Res. 2, 501–514 (2021).
Biskup, T. et al. Direct observation of a photoinduced radical pair in a cryptochrome blue-light photoreceptor. Angew. Chem. Int. Ed. 48, 404–407 (2009).
Hasegawa, M. et al. Regulated electron tunneling of photoinduced primary charge-separated state in the photosystem II reaction center. J. Phys. Chem. Lett. 8, 1179–1184 (2017).
Closs, G. L., Forbes, M. D. E. & Norris, J. R. Spin-polarized electron-paramagnetic resonance-spectra of radical pairs in Micelles - Observation of Electron-Spin Spin Interactions. J. Phys. Chem. 91, 3592–3599 (1987).
Hore, P. J., Hunter, D. A., McKie, C. D. & Hoff, A. J. Electron paramagnetic resonance of spin-correlated radical pairs in photosynthetic reactions. Chem. Phys. Lett. 137, 495–500 (1987).
Avdievich, N. I. & Forbes, M. D. E. Dynamic effects in spin-correlated radical pair theory: J modulation and a new look at the phenomenon of alternating line widths in the EPR spectra of flexible biradicals. J. Phys. Chem. 99, 9660–9667 (1995).
Savitsky, A., Dubinskii, A. A., Flores, M., Lubitz, W. & Mobius, K. Orientation-resolving pulsed electron dipolar high-field EPR spectroscopy on disordered solids: I. Structure of spin-correlated radical pairs in bacterial photosynthetic reaction centers. J. Phys. Chem. B 111, 6245–6262 (2007).
van der Est, A., Bittl, R., Abresch, E. C., Lubitz, W. & Stehlik, D. Transient Epr spectroscopy of perdeuterated Zn-substituted reaction centers of rhodobacter-sphaeroides R-26. Chem. Phys. Lett. 212, 561–568 (1993).
Kobori, Y., Ako, T., Oyama, S., Tachikawa, T. & Marumoto, K. Transient electron spin polarization imaging of heterogeneous charge-separation geometries at bulk-heterojunction interfaces in organic solar cells. J. Phys. Chem. C. 123, 13472–13481 (2019).
Kobori, Y., Noji, R. & Tsuganezawa, S. Initial molecular photocurrent: nanostructure and motion of weakly bound charge-separated state in organic photovoltaic interface. J. Phys. Chem. C. 117, 1589–1599 (2013).
Miura, T., Aikawa, M. & Kobori, Y. Time-resolved EPR study of electron-hole dissociations influenced by alkyl side chains at the photovoltaic polyalkylthiophene:PCBM interface. J. Phys. Chem. Lett. 5, 30–35 (2014).
Kobori, Y., Sekiguchi, S., Akiyama, K. & Tero-Kubota, S. Chemically induced dynamic electron polarization study on the mechanism of exchange interaction in radical ion pairs generated by photoinduced electron transfer reactions. J. Phys. Chem. A 103, 5416–5424 (1999).
Scott, A. M. et al. Spin-selective charge transport pathways through p-oligophenylene-linked donor bridge-acceptor molecules. J. Am. Chem. Soc. 131, 17655–17666 (2009).
Kobori, Y. et al. Superexchange electron tunneling mediated by solvent molecules: Pulsed electron paramagnetic resonance study on electronic coupling in solvent-separated radical ion pairs. J. Phys. Chem. B 108, 10226–10240 (2004).
Kobori, Y. & Fuki, M. Protein–ligand structure and electronic coupling of photoinduced charge-separated state: 9,10-anthraquinone-1-sulfonate bound to human serum albumin. J. Am. Chem. Soc. 133, 16770–16773 (2011).
Fuki, M., Murai, H., Tachikawa, T. & Kobori, Y. Time resolved EPR study on the photoinduced long-range charge-separated state in protein: electron tunneling mediated by arginine residue in human serum albumin. J. Phys. Chem. B 120, 4365–4372 (2016).
Regev, A., Michaeli, S., Levanon, H., Cyr, M. & Sessler, J. L. Solvent effect in randomly and partially oriented triplets of the sapphyrin dication: optical and fast EPR-magnetophotoselection measurements. J. Phys. Chem. 95, 9121–9129 (1991).
Biskup, T. “Elektronenspinresonanz an funktionell relevanten paramagnetischen Intermediaten blaulichtaktiver Flavoproteine” Docter thesis, Freie Universität Berlin, (2009).
Miura, T. et al. Geometries, electronic couplings, and hole dissociation dynamics of photoinduced electron–hole pairs in polyhexylthiophene–fullerene dyads rigidly linked by oligophenylenes. J. Am. Chem. Soc. 138, 5879–5885 (2016).
Kobori, Y., Ponomarenko, N. & Norris, J. R. Time-resolved electron paramagnetic resonance study on cofactor geometries and electronic couplings after primary charge separations in the photosynthetic reaction center. J. Phys. Chem. C. 119, 8078–8088 (2015).
Fukuju, T., Yashiro, H., Maeda, K., Murai, H. & Azumi, T. Singlet-born SCRP observed in the photolysis of tetraphenylhydrazine in an SDS micelle: time dependence of the population of the spin states. J. Phys. Chem. A 101, 7783–7786 (1997).
Miura, T. & Murai, H. Effect of molecular diffusion on the spin dynamics of a micellized radical pair in low magnetic fields studied by monte carlo simulation. J. Phys. Chem. A 119, 5534–5544 (2015).
Eaton, W. A., Hofrichter, J., Makinen, M. W., Andersen, R. D. & Ludwig, M. L. Optical spectra and electronic structure of flavine mononucleotide in flavodoxin crystals. Biochemistry 14, 2146–2151 (1975).
Kay, C. W. M., Bittl, R., Bacher, A., Richter, G. & Weber, S. Unambiguous determination of the g-Matrix orientation in a neutral flavin radical by pulsed electron−nuclear double resonance at 94 GHz. J. Am. Chem. Soc. 127, 10780–10781 (2005).
Okafuji, A., Schnegg, A., Schleicher, E., Möbius, K. & Weber, S. G-Tensors of the flavin adenine dinucleotide radicals in glucose oxidase: a comparative multifrequency electron paramagnetic resonance and electron−nuclear double resonance study. J. Phys. Chem. B 112, 3568–3574 (2008).
Bleifuss, G. et al. Tryptophan and tyrosine radicals in ribonucleotide reductase: a comparative high-field EPR study at 94 GHz. Biochemistry 40, 15362–15368 (2001).
Sjödin, M. et al. Switching the redox mechanism: models for proton-coupled electron transfer from tyrosine and tryptophan. J. Am. Chem. Soc. 127, 3855–3863 (2005).
Pogni, R. et al. A tryptophan neutral radical in the oxidized state of versatile peroxidase from Pleurotus eryngii - A combined multifrequency EPR and density functional theory study. J. Biol. Chem. 281, 9517–9526 (2006).
Lhoste, J. M., Haug, A. & Hemmerich, P. Electron paramagnetic resonance studies of the triplet state of flavin and pteridine derivatives. Biochemistry 5, 3290–3300 (1966).
Kay, C. J., Barber, M. J., Notton, B. A. & Solomonson, L. P. Oxidation–reduction midpoint potentials of the flavin, haem and Mo-pterin centres in spinach (Spinacia oleracea L.) nitrate reductase. Biochem. J. 263, 285–287 (1989).
Medvedev, E. S. et al. Protein dynamics control of electron transfer in photosynthetic reaction centers from Rps. sulfoviridis. J. Phys. Chem. B 112, 3208–3216 (2008).
Mcconnell, H. Intramolecular charge transfer in aromatic free radicals. J. Chem. Phys. 35, 508–515 (1961).
Sjulstok, E., Olsen, J. M. H. & Solov’yov, I. A. Quantifying electron transfer reactions in biological systems: what interactions play the major role? Sci Rep-Uk. 5, (2015).
Tominaga, K., Walker, G. C., Jarzeba, W. & Barbara, P. F. Ultrafast charge separation in adma - experiment, simulation, and theoretical issues. J. Phys. Chem. 95, 10475–10485 (1991).
Fukuju, T., Yashiro, H., Maeda, K. & Murai, H. Real-time observation of the singlet–triplet dephasing effect on the spin dynamics of the spin-correlated radical pair formed in the photolysis of tetraphenylhydrazine in a micelle. Chem. Phys. Lett. 304, 173–179 (1999).
Zoltowski, B. D. et al. Chemical and structural analysis of a photoactive vertebrate cryptochrome from pigeon. Proc. Natl. Acad. Sci. U. S. A. 116, 19449–19457 (2019).
Di Valentin, M. et al. Evidence for water-mediated triplet–triplet energy transfer in the photoprotective site of the peridinin–chlorophyll a–protein. Biochimica et. Biophysica Acta (BBA) - Bioenerg. 1837, 85–97 (2014).
Miller, J. R., Beitz, J. V. & Huddleston, R. K. Effect of free energy on rates of electron transfer between molecules. J. Am. Chem. Soc. 106, 5057–5068 (1984).
Hoshino, M., Arai, S. & Imamura, M. Low-temperature pulse radiolysis. II. Time-Depend. spectra anions Aromat. ketones. J. Phys. Chem. 78, 1473–1477 (1974).
Huddleston, R. K. & Miller, J. R. Dynamic spectral shifts of molecular anions in organic glasses. J. Phys. Chem. 86, 2410–2415 (1982).
Chakrabarti, S., Parker, M. F. L., Morgan, C. W., Schafmeister, C. E. & Waldeck, D. H. Experimental evidence for water mediated electron transfer through bis-amino acid donor−bridge−acceptor oligomers. J. Am. Chem. Soc. 131, 2044–2045 (2009).
Maeyama, T., Yagi, I., Yoshida, K., Fujii, A. & Mikami, N. Photodetachment spectroscopy of fluorenone radical anions microsolvated with methanol: rationalizing the anomalous solvatochromic behavior due to hydrogen bonding. J. Phys. Chem. A 119, 3721–3730 (2015).
Bellissent-Funel, M.-C. et al. Water determines the structure and dynamics of proteins. Chem. Rev. 116, 7673–7697 (2016).
Fogarty, A. C. & Laage, D. Water dynamics in protein hydration shells: the molecular origins of the dynamical perturbation. J. Phys. Chem. B 118, 7715–7729 (2014).
Tamura, H. & Burghardt, I. Ultrafast charge separation in organic photovoltaics enhanced by charge delocalization and vibronically hot exciton dissociation. J. Am. Chem. Soc. 135, 16364–16367 (2013).
Beratan, D. N., Betts, J. N. & Onuchic, J. N. Protein electron-transfer rates set by the bridging secondary and tertiary structure. Science 252, 1285–1288 (1991).
Balabin, I. A., Hu, X. Q. & Beratan, D. N. Exploring biological electron transfer pathway dynamics with the Pathways Plugin for VMD. J. Comput. Chem. 33, 906–910 (2012).
Balabin, I. A. & Onuchic, J. N. Dynamically controlled protein tunneling paths in photosynthetic reaction centers. Science 290, 114–117 (2000).
Kobori, Y. & Miura, T. Overcoming coulombic traps: geometry and electronic characterizations of light-induced separated spins at the bulk heterojunction interface. J. Phys. Chem. Lett. 6, 113–123 (2015).
Hoshina, H., Kanemura, T. & Ruggiero, M. T. Exploring the dynamics of bound water in nylon polymers with terahertz spectroscopy. J. Phys. Chem. B 124, 422–429 (2020).
Liscum, E., Hodgson, D. W. & Campbell, T. J. Blue light signaling through the cryptochromes and phototropins. so that’s what the blues is all about. Plant Physiol. 133, 1429–1436 (2003).
Turton, D. A. et al. Terahertz underdamped vibrational motion governs protein-ligand binding in solution. Nat. Commun. 5, 3999 (2014).
Daiyasu, H. et al. Identification of cryptochrome DASH from vertebrates. Genes Cells 9, 479–495 (2004).
Yamada, D. et al. Fourier-transform infrared study of the photoactivation process of Xenopus (6–4) photolyase. Biochemistry 51, 5774–5783 (2012).
Biskup, T. et al. Unexpected electron transfer in cryptochrome identified by time-resolved EPR. Spectrosc. Angew. Chem. -Int. Ed. 50, 12647–12651 (2011).
Payne, G., Wills, M., Walsh, C. & Sancar, A. Reconstitution of Escherichia coli photolyase with flavins and flavin analogues. Biochemistry 29, 5706–5711 (1990).
Edeson, S. J. et al. Studies on the stereochemical assignment of 3-acylidene 2-oxindoles. Org. Biomol. Chem. 12, 3201–3210 (2014).
Burzio, L. A. & Waite, J. H. Reactivity of peptidyl-tyrosine to hydroxylation and cross-linking. Protein Sci. 10, 735–740 (2001).
Matsuda, S., Oyama, S. & Kobori, Y. Electron spin polarization generated by transport of singlet and quintet multiexcitons to spin-correlated triplet pairs during singlet fissions. Chem. Sci. 11, 2934–2942 (2020).
Kobori, Y. et al. Primary Charge-Recombination in an Artificial Photosynthetic Reaction Center. Proc. Natl. Acad. Sci. U. S. A. 102, 10017–10022 (2005).
Norris, J. R., Morris, A. L., Thurnauer, M. C. & Tang, J. A General-Model of Electron-Spin Polarization Arizing From the Interactions within Radical Pairs. J. Chem. Phys. 92, 4239–4249 (1990).
Morris, A. L. et al. Electron-spin Polarization Model Applied to Sequential Electron-Transfer in Iron-Containing Photosynthetic Bacterial Reaction Centers with Different Quinones as QA. J. Phys. Chem. 99, 3854–3866 (1995).
Acknowledgements
This work was partially supported by JSPS KAKENHI Grant Numbers of 19H00888, 20K21174 and Grant-in-Aid for Transformative Research Areas, “Dynamic Exciton” (JP20H05832) to Y.K. Y.K. appreciate the support by Dr. Hiroki Nagashima (Saitama University) in the TREPR measurements using the cryostat system. YK thanks Professor Kiminori Maeda (Saitama University) for fruitful discussions on the transient intermediate species during the RPs.
Author information
Authors and Affiliations
Contributions
Y.K. and S.W. conceived the project. T.I. and H.K. expressed the proteins and characterized the purifications. M.H., S.W., M.F., and Y.K. performed TREPR measurements. Y.K. developed the theoretical description and the MATLAB codes for analyzing the TREPR data. Y.K., T.I., and S.W. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Chemistry thanks Malcolm Forbes, Marco Chino and Daniel Kattnig for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamada, M., Iwata, T., Fuki, M. et al. Orientations and water dynamics of photoinduced secondary charge-separated states for magnetoreception by cryptochrome. Commun Chem 4, 141 (2021). https://doi.org/10.1038/s42004-021-00573-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-021-00573-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.