Abstract
Hydrogenation is an effective approach to improve the performance of photocatalysts within defect engineering methods. The mechanism of hydrogenation and synergetic effects between hydrogen atoms and local electronic structures, however, remain unclear due to the limits of available photocatalytic systems and technical barriers to observation and measurement. Here, we utilize oxygen vacancies as residential sites to host hydrogen atoms in a layered bismuth oxychloride material containing defects. It is confirmed theoretically and experimentally that the hydrogen atoms interact with the vacancies and surrounding atoms, which promotes the separati30on and transfer processes of photo-generated carriers via the resulting band structure. The efficiency of catalytic activity and selectivity of defective bismuth oxychloride regarding nitric oxide oxidation has been improved. This work clearly reveals the role of hydrogen atoms in defective crystalline materials and provides a promising way to design catalytic materials with controllable defect engineering.
Similar content being viewed by others
Introduction
Semiconducting photocatalysts have attracted tremendous attention due to their capability of utilizing solar light to drive chemical reactions, including water splitting1, carbon dioxide reduction2,3,4 and pollutant degradation5. Their performances are determined by electronic structures, surface states and morphologies. The former determines the key charge carrier dynamics in photocatalytic processes including photoexcited charge carrier generation, separation and transport, while the latter dominates the photocatalytic activities. Hydrogenation has been proven to be a promising approach to modify semiconductor-based photocatalysts though modulating physical and chemical characteristics6. It can create point defects in photocatalysts that enhance absorption of light by generating impurity bands in the energy gap7,8,9. The defects may also lead to a local electric field that promotes photoexcited charge carrier separation and modified transfer rates, which ultimately enables photocatalysts to achieve excellent catalytic performance10,11,12. In addition, the disordered surface structures induced by hydrogenation can act as active sites for energy conversion reactions7,8,9. As an example, hydrogenation can induce high-density oxygen vacancies in oxide photocatalysts, which facilitates photocatalytic water splitting by broadening the light absorption spectra and modifying the redox ability of photoexcited charge carriers13,14,15. For example, the TiO2 will become yellow or black after hydrogenation and exhibits significantly improved solar photocatalytic performances, and also possess excellent photoelectrochemical–water-splitting performance13,14. Recently, theoretical studies revealed that hydrogen can be easily captured by vacancy sites in semiconductors because vacancies always possess high surface energy16,17. Protons (H+) can be captured by defects and converted into negatively ionized hydride ions (H−) by migration of electrons, which possibly enhance conductivity and local reactivity of such semiconductors18,19. These studies imply that hydrogenation may promote photocatalytic performance of semiconductors not only by creating defects but also through hydrogen doping at these defect sites. Nevertheless, the experimental examination of this hypothesis is limited by technical barriers to visualizing atomic positions and the chemical dynamics of doped hydrogen atoms/ions due to their small atomic radius as well as small atomic mass20,21. Whether the doped hydrogen would improve photocatalytic performance, therefore, remains in argument and limits full understanding of hydrogenation mechanisms on photocatalysis.
Bismuth oxychloride BiOCl, a conventional semiconductor photocatalyst, possesses a unique two-dimensional (2D) layered structure, in which the [Bi2O2]2+ slabs are interleaved with double slabs of halogen ions. This special layered structure can establish an internal electric field to inhibit the recombination of photogenerated charge carriers. This enables BiOCl to efficiently degrade pollutants, decompose water to generate oxygen and reduce the carbon dioxide to carbon monoxide22,23. In our previous work, it has been proven that oxygen vacancies can be created easily and are energetically stable, because the material can easily produce a low density of dangling bonds on their surface, which then results in a number of defects. Interestingly, the size of the vacancies are large enough for anchoring and hosting heteroatoms, with hydrogen atoms/ions24,25. The hydrogen dopants are likely to be introduced into BiOCl oxygen vacancies by standard hydrogenation approaches. It is, therefore, expected to reveal the role of doping hydrogen atoms by using defective BiOCl as a chemical probe.
In this work, we dope defective BiOCl nanosheets with hydrogen heteroatoms by a low-temperature hydrogeneration method. The hydrogen atoms are successfully anchored at defect sites, which is verified by spectral studies. The NO oxidation activity and selectivity of defective BiOCl are significantly enhanced after hydrogeneration. The experimental characterization and theoretical simulation clearly verify that the enhancements of photocatalytic performance are attributable to modulation of energy dispersion by impurity bands, which leads to both improvements in charge separation and photocatalytic activity. This work not only confirms the role of hydrogen heteroatoms in photocatalysts, but also offers a possible way to achieve high-performance photocatalysts via hydrogen doping.
Results and discussion
Synthesis and basic characterization of catalyst
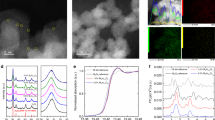
Hydrogenated BiOCl nanoparticles with oxygen vacancies (OV) (H-BiOCl OV) were obtained by annealing BiOCl OV nanoparticles under Ar and H2 atmosphere respectively (experimental details in the supporting information). The morphological feature of as-prepared H-BiOCl OV was characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM), as shown in Supplementary Fig. 1, which indicates that the nanoparticles structure shows no apparent change after hydrogenation and no structural disorder or clusters have been detected. The comparison of X-ray diffraction (XRD) patterns (Supplementary Fig. 2) demonstrate that the H-BiOCl OV was of high purity with orientation along the [001] direction (JCPDS card no. 73-2049), indicating that the nanoparticle crystal structure was not changed after hydrogenation. In the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image (Fig. 1b), we can clearly see the high crystallinity of the nanoparticles and the interlayer distance is about 0.76 nm (d001) in H-BiOCl OV, which is very similar to BiOCl OV (0.78 nm). These results suggest the hydrogen atoms have diffused into the oxygen vacancies and settled down with the vacancies; meanwhile, structural distortion or further disorder is not expected, as illustrated in the schematic diagram in Fig. 1a.
Furthermore, the effect of hydrogen atoms on the chemical bonding, surface compositions and light absorption is examined. As shown in Supplementary Fig. 3a, b, compared to the BiOCl OV, the UV−Vis diffuse reflectance spectrum of the H-BiOCl OV shows stronger visible light absorption and a small redshift in extrapolated absorption edge. This suggests that the oxygen vacancies that induce the visible light absorption of BiOCl OV have been effectively mediated by hydrogen atoms23,25,26. This is also confirmed by the electron paramagnetic resonance (EPR) measurement, which can probe the electrons trapped in the oxygen vacancies23,27. As shown in Fig. 1c, the BiOCl OV exhibited a strong EPR signal at g = 2.004 than H-BiOCl OV, indicating that H-BiOCl OV traps a lower number of electrons in oxygen vacancies2. At the same time, as illustrated by the O1S X-ray photoelectron spectroscopy (XPS) spectra in Fig. 1d, the peak at 531.13 eV could be ascribed to surface hydroxyls oxygen atoms, which is in the shadow of oxygen vacancies.
The peak of H-BiOCl OV exhibits a 0.15 eV blue-shift, which means the electrons around the oxygen atoms have been reduced due to the inductive effect of hydrogen atoms. According to the peak area at 531.13 eV of O1s, we can conclude that the hydrogen atoms could result in oxygen vacancies being reduced and the number of surface hydroxyl oxygens decreased2,24. Meanwhile, the distribution of electrons results in the Bi4f XPS peaks of H-BiOCl OV being blue shifted by 0.23 eV compared with BiOCl OV (Fig. 1e). This is further confirmed by extended X-ray absorption fine structure (EXAFS) spectroscopy. The EXAFS spectra of Bi L3-edge were further performed to investigate the valence and coordination states of Bi in samples28. Supplementary Fig. 4 shows the absorption edge of H-BiOCl OV moves to lower energy than BiOCl OV, indicating that the lower states of Bi appear. In the Fourier transform spectra of the Bi L3-edge EXAFS oscillations (Fig. 2b), we can see there is no shift of Bi-O at the sharp peaks around 1.6 Å, but the peak of Bi-Bi at 3.8 Å in H- BiOCl OV is shorter than in BiOCl OV. It indicates that there are a number of localized electrons around Bi atoms and the orbitals of hydrogen atoms simultaneously form a hydrazide with bismuth atoms25, which validates the localized charge density around vacancy sites being decreased due to the presence of the hydrogen atoms.
Structural characterization of hydrogen-modified defective BiOCl
The location of hydrogen atoms in H-BiOCl OV was studied by solid-state nuclear magnetic resonance spectroscopy (ssNMR) measurements, which can confirm the location of hydrogen atoms in our sample. Figure 2a shows the 1H solid-state NMR spectra of BiOCl OV and H-BiOCl OV. The main peak at the chemical shift of 3.78 ppm is about the main type of bridging proton on the surface, which can be assigned to the terminal hydroxyl groups (OHT) and water molecules associated with the surface sites29,30. The slightly larger peak at 4.52 ppm and 4.76 ppm is surface-adsorbed water31. It is worth noting that an additional small peak centered at the chemical shift of 6.68 ppm is observed in H-BiOCl OV. It can be assigned to the 1H signal of bridging hydroxyl groups (OHB) in hydrogen-bonding interaction with surface-adsorbed H2O, which is the crucial group to affect the photocatalytic reaction process29. The minor peak around 0.8 ppm corresponds to hydrogen atoms with a weakened shielding effect from their surrounding environment, and these can be attributed to internal bridging hydrogen atoms (Bi-H-Bi) where the hydrogen atoms occupy the oxygen vacancies32. At the same time, H-BiOCl OV has a minor peak at 1.31 ppm, which can be associated with a typical chemical shift region for the terminal hydrogen atoms (OHT)32. These two peaks are probably related, with the hydrogen atoms located in the more complicated environments, suggesting that the hydrogen atoms‘ mobility in H-BiOCl OV is higher than that of BiOCl OV. It agrees with the Fourier transform infrared (FT-IR) spectroscopy.
As shown in Supplementary Fig. 5, both materials show similar absorption features from 500 to 4000 cm−1, with an absorption peak at 1604 cm−1 in the FTIR spectrum ascribed to bending vibrations of free water molecules, signifying isolated hydroxyl groups with O-H deformation vibrations. The peaks at around 3470 cm−1 are associated with the O–H stretch of intermolecular hydrogen bonds due to O–H stretching and wagging modes6,33. The intensity of absorption band around 3400 cm−1 of H-BiOCl OV is weaker and wider than BiOCl OV, which indicates that the hydrogen incorporated into the BiOCl OV possibly does not passivate a significant number of OH dangling bonds as this would otherwise increase the absorption30. This suggests that the OH groups experience a more varied environment on the H-BiOCl OV surface rather than on the surface of the BiOCl OV.
In order to get further understanding of the hydrogen atoms in the sample, we obtained Raman spectra of BiOCl OV and H-BiOCl OV (Fig. 2c). The peaks at 60.9 and 136.25 cm−1 can be ascribed to the A1g internal Bi−Cl stretching and the Eg external stretching modes of Bi−Cl25. It has been widely recognized that the lattice periodicity and symmetry breakage can induce new vibration modes. As a result of the existence of oxygen vacancies in the materials, there is a new peak at 99.2 cm−1 that appears in the spectra of BiOCl OV and H-BiOCl OV, which corresponds to A1g phonon modes of rhombohedral Bi34. Meanwhile, the new peak at 116.3 cm−1 appears in the spectra of H-BiOCl OV. This cannot be ascribed to any ordinary Raman bands of BiOCl, and may originate from surface or intersurface vibration modes induced by the hydrogen atoms35. Compared with the NMR result we think this new peak may relate to the interaction between hydrogen atoms and bismuth atoms.
Density functional theory calculations and experiment characterization
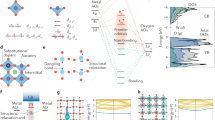
To uncover the effect of hydrogen atom in mediating the vacancies and the electronic structures, density functional theory (DFT) calculations were carried out. As shown in Fig. 3a, b, the valence band maximum (VBM) of H-BiOCl OV is upshifted to the Fermi level (Ef = 0 eV) than BiOCl OV, with both samples showing indirect band gaps. The electronic density of states (DOS) clearly suggests that the new electronic states between the gap of the BiOCl OV are mainly created by hybridization of occupied Bi-6p, Cl-3p, and O-2p orbitals (Fig. 3c, d). But in the H-BiOCl OV, the defect states are mainly composed of the hybridization of Bi-6p and H-1s. Meanwhile, we have obtained the reaction energy path of the hydrogen atom diffusion into the BiOCl OV. The detailed atomic configurations of the initial state, intermediate states, and the final state are also plotted in Supplementary Fig. 6. We found that the hydrogen atom only needs to overcome an activation energy barrier around 0.15 eV from the free standing BiOCl OV surface to migrate to a position which is near to the oxygen defect location. The final energy state is 1.75 eV lower than the initial state. The charge density of the defect state analysis indicates that electrons move from OVs to the neighboring Bi and O atoms in the BiOCl OV (Fig. 3a, inset image). When an H atom stays on the site of OV, the neighboring Bi and O atoms give electrons to the H atom, making the hydrogen atom negatively charged (Fig. 3b, inset image). More importantly, as shown in Supplementary Fig. 7, the charge density difference map shows the displacement of electronic charge induced by the interaction of the H atom with its Bi neighbor. It indicates that charge accumulates on the H atom and is depleted on the neighboring Bi atoms, and the overlapping of charge density of hydrogen and the closest O atoms can be observed. This implies that there is a bonding cloud between H atom and Bi atoms. These results agree well with the experimental results presented above.
Band structure of BiOCl OV (a) and H-BiOCl OV (b), which gives the corresponding charge densities of defect energy levels as inserts and the pink, green, blue colors in bands represent the contributions of Bi6p, Cl3p, H1s orbitals. Density of states (DOS) of BiOCl OV (c) and H-BiOCl OV (d), the enlarged inserts are the PDOS of defect states. Red, orange, blue, pink lines are Bi6p, Cl3p, O2p and H1s orbitals.
Additionally, from the above theoretical simulations, we can see that the hydrogen atoms can affect the material band structure. Through a plot of the transformed Kubelka–Munk function versus light energy (Supplementary Fig. 3b), the bandgap of H-BiOCl OV is 2.88 eV, which is narrower than BiOCl OV (2.99 eV). The XPS valance band spectra (Supplementary Fig. 8a) and Mott–Schottky plot (Supplementary Fig. 8b) were employed to directly pinpoint the valence band position of the semiconductor. In detail, the VBM and the flat band potential of H-BiOCl OV is upshifted from BiOCl OV. As displayed in Supplementary Fig. 9a, the optical fluorescence spectra of BiOCl OV exhibits two main peaks centered at 434 and 514 nm. Those two main peaks come from the intrinsic band-edge and defect state emissions. Comparing with the BiOCl OV, the second peak’s position of H-BiOCl OV is red-shifted and the ratio of two main peak areas becomes bigger. This means the intermediate states in the bandgap of H-BiOCl OV are upshifted and broader than BiOCl OV. The schematic illustration of the band structure of BiOCl OV and H-BiOCl OV has been shown in Supplementary Fig. 9b, which indicates that the VBM of H-BiOCl OV is much more positive than BiOCl OV, which will make it energetically feasible for the trapped electrons to generate O2•‒. The resulting defect trap states can allow the electrons to be easily photoexcited to the conduction band (CB) from valence band (VB) and transferred to the surface. Hence, the electrons will be abler to activate O2 to O2•‒ and OH to OH•. Furthermore, the electron spin resonance (ESR) spectra (Supplementary Fig. 10) suggest that H-BiOCl OV will possess a better ability than BiOCl OV to activate O2 to O2•‒ after visible light irradiation, implying that the induced hydrogen atoms can enhance charge carrier yields.
To further understand the effect of hydrogen atom-occupied vacancies on the charge separation inside the semiconductors, we performed photoluminescence measurements. The time-resolved fluorescence emission decay spectra are shown in Fig. 2d, indicating that the H-BiOCl OV exhibited the longer lifetime of (τ1 = 1656.56 ps, 1.95%, τ2 = 277.00 ps, 97.40%, τ3 = 6019.14 ps, 6.3%, average lifetime ~37.44 ps) than BiOCl OV (τ1 = 2016.77 ps, 7.57%, τ2 = 248.40 ps, 98.99%, τ3 = 6803.89 ps, 2.47%, average lifetime ~16.81 ps). In addition, the sample exhibits a transient photoresponse to visible light, as shown in Supplementary Fig. 11a, which clearly indicates that the H-BiOCl OV can generate more photoinduced charge carried by absorbing visible light than BiOCl OV. Moreover, it is interesting to find that the arc radius on the EIS Nyquist plot (Supplementary Fig. 11b) of H-BiOCl OV is smaller than the arc radius of BiOCl OV under visible light irradiation, which suggests that the H-BiOCl OV has higher efficiency in separating and transferring photogenerated electron−hole pairs among the interface. Accordingly, using the hydrogen atom-mediated vacancies can reduce the photogenerated recombination rate and increase the ability of redox reactions.
Photocatalysts performance characterization
To verify the above supposition and shed light on the effect of hydrogen atom introduced into the oxygen vacancies on the photocatalytic process, photocatalytic nitric oxide (NO) oxidation of both BiOCl OV and H-BiOCl OV was measured under visible light (λ > 420 nm) from a 100 mW commercial tungsten halogen lamp. As depicted in Fig. 4a, the H-BiOCl OV reaches a high NO removal ratio of 45% after irradiation for 30 min, which is about 13% more efficient than BiOCl OV. In addition, the corresponding conversion rates of H-BiOCl OV for generating NOX− is much greater than BiOCl OV (Supplementary Fig. 12) in the whole catalytic process. As shown in Supplementary Fig. 13, H-BiOCl OV can be efficiently reused for NO removal with good recyclability and stability, which did not show any loss of photocatalytic activity, and the XRD patterns of reused H-BiOCl OV (Supplementary Fig. 14) do not show any obvious variation after cycling photooxidation test. These results clearly show that H-BiOCl OV exhibit good photocatalytic recyclability and stability under visible light.
a Photocatalytic removal of NO in the presence of BiOCl OV and H-BiOCl OV under illumination by visible light (λ > 420 nm). b In situ FTIR spectra of the photocatalytic NO oxidation process over H-BiOCl-OV under simulated solar-light irradiation. c In-situ FTIR spectra of the NO adsorption process (d) and degradation process. e Corresponding normalized absorbance radio between ν (•NO3−) 1003 cm−1 and ν (NO2) 1725 cm−1.
To further uncover the underlying reasons for the enhancement of NO removal efficiency by H-BiOCl OV over BiOCl OV, in situ FT-IR spectroscopy measurements were performed on two samples of each material. The in situ FTIR spectra (Supplementary Fig. 15) show that the absorption peaks of NO (963, 1612 cm−1)36, NO2 (1443 cm−1)37 and NO2− (1174 cm−1)38 appear on the surface of both H-BiOCl OV and BiOCl OV after they adsorb NO in the dark for 20 min. The intensity of all absorption bands on the surface of H-BiOCl OV is stronger than BiOCl OV (Fig. 4c). From the in situ FTIR spectra of photocatalytic reaction processes on the surface of H-BiOCl OV (Fig. 4b) and BiOCl OV (Supplementary Fig. 16a) under visible light irradiation, we see that new absorption bands of NO2 (at 1725 cm−1)39 and nitrates (at 809, 1275 and 1494 cm−1)40 appear and increase during the whole light irradiation process (Fig. 4d), indicating that an amount of nitrite and nitrate ions is produced on the catalyst surface.
It is worth noting that the strong characteristic peak intensity of •O2− radicals at 1003 cm−1 is typically indicated on the surface of the samples41. The absorption intensity of this intermediate product has been normalized (Supplementary Fig. 16b). The change trends of this reactive oxygen species on the surface of BiOCl OV and H-BiOCl OV are similar. The final absorption intensity of •O2− by H-BiOCl OV is stronger than that of BiOCl OV; therefore, the O2 molecules adsorbed on the surface of H-BiOCl OV are more easily activated and can receive more electrons to convert to •O2− radicals that adsorb on the surface of BiOCl OV. This reactive oxygen species participates in photocatalytic redox reaction, which ultimately enhances the efficiency of the photocatalytic reaction.
At the same time, the absorption bands located at 2162 cm−1 (NO+)42 appear on the surface of both these sample types. The normalized absorption of this product reveals that the relative content of NO+ on the surface of H-BiOCl OV is larger than on BiOCl OV (Supplementary Fig. 16c). Moreover, we found the 1673 cm−1 absorption band (NO2+)43 only appears on the surface of H-BiOCl OV (Fig. 4b). Those two products are derived from oxidation of NO and NO2 by •OH and •O2−, then final oxidation into the nitrites or nitrates. In addition, NO2 is a toxic air pollutant, so the conversion of NO by oxidation into NO2 should be inhibited. Comparing the normalized absorption intensity of the NO3− (1274 cm−1) and NO2 (1725 cm−1), the proportions on the trend chart of Fig. 4e indicate that the H-BiOCl OV produces more NO3− rather than NO2. Overall, these results demonstrate that utilizing hydrogen atoms to modify oxygen vacancies can directly and immediately enhance the reactive efficiency and selectivity of BiOCl OV.
The possible reaction mechanism of NO photocatalytic oxidation by H-BiOCl OV we propose is given in the Supplementary Discussion.
In summary, we successfully introduced hydrogen atoms into a defective BiOCl crystal structure by utilizing the oxygen vacancies. By integrating the results of the experiments and theoretical calculations, we have demonstrated the role of hydrogen atoms in the BiOCl lattice with O vacancy defects. The hydrogen atoms show a preference to occupy the oxygen vacancies’ states and hybridize with the nearby atoms. This results in generation of new trap states among the impurity states and upshifts the position of the valence band. This effect improves the redox reactivity and selectivity efficiency of BiOCl OV for solar-driven NO oxidation by improving the separation and transfer efficiency of photogenerated carriers. This study provides distinct insights exploring the role of hydrogen atoms in oxide materials having defects. Importantly, this work not only provides a feasible plan for designing high-efficiency photocatalysts through introduction of simple elements, but also sheds light on the crucial role of elemental doping in photocatalysts at the atomic level.
Methods
Catalyst characterization
Powder XRD patterns were collected via an X-ray diffractometer (GBC MMA diffractometer) with Cu Ka radiation and a working voltage of 40 kV. The morphologies of the as-prepared samples were characterized by SEM (JEOL JSM-7500FA). The details of the crystal structure were further examined by STEM (JEOL JEM-ARM 200 F, operating at 200 kV). UV−Vis diffuse reactance spectra were collected on UV–Vis–NIR spectrophotometer (UV-3600, Shimadzu) using 100% BaSO4 as the reference sample. X-ray spectroscopy (XPS) and X-ray absorption spectra (XAS) were conducted at the Photoelectron Spectroscopy Station (Beamline 4W9B) of the Beijing Synchrotron Radiation Facility of the Institute of High Energy Physics, Chinese Academy of Sciences. EPR and ESR spectroscopy were conducted on a JES FA-200 spectrometer. FT-IR spectrometry was obtained from a Shimadzu FTIR Prestige-21. The Raman spectra were obtained by the Nanofinder system. The 1H NMR spectra were acquired on a Bruker Advance III 500WB spectrometer. Photoluminescence data were collected using a Jobin Hvon Flurolog 3 from Horiba, with a Xenon arc lamp as a light source and either PMT or InGaAs detector for visible and NIR collection, respectively. The time-resolved phosphorescence spectra were collected using a 334 nm Nano LED source as the examination light.
Photoelectrochemical characterization of catalyst
A three-electrode cell was used to carry out the electrochemical measurements on an electrochemical workstation (CHI-660D, China). The working electrode was a catalyst-coated FTO electrode. An Ag/AgCl electrode was used as the reference electrode. A Pt wire was used as the counter electrode and saturated 0.1 M Na2SO4 solution was used as the electrolyte. A 300 W Xe lamp with a cut-off filter (λ > 420 nm) was used as the light source. The Mott−Schottky measurements were monitored at a fixed frequency of 100 Hz with 10 mV amplitude at various potentials.
Photocatalytic reactions and in situ FTIR investigation
The photocatalytic activity was evaluated based on the removal efficiency of NO at ppb levels in a continuous flow reactor with 0.2 g prepared sample. The concentration of NO was continuously detected by an NOx analyzer (42c-TL, Thermo Environmental Instruments Inc.). A 150 W commercial Xenon lamp with a 420 nm cut-off filter that was vertically placed above the reactor glowed when the adsorption–desorption equilibrium was achieved. The quantum efficiencies of NO oxidation at a variety of wavelengths were measured by inserting monochromatic filters in front of the reactor. In situ FTIR measurements were conducted using a TENSOR II FT-IR spectrometer (Bruker) equipped with an in situ diffuse reflectance cell (Harrick) and a high-temperature reaction chamber.
The reaction chamber was equipped with three gas ports and two coolant ports. High-purity He, high-purity O2, and a mixture of 100 ppm of NO in He could be fed into the reaction system, and a three-way ball valve was used to switch between the target gas (NO) and the purge gas (He). The total gas flow rate was 100 mL min−1, and the concentration of NO was adjusted to 50 ppm by dilution with O2. The chamber was enclosed with a dome having three windows, two for IR light entrance and detection, and one for illuminating the photocatalyst. The observation window was made of UV-absorbing quartz and the other two windows were made of ZnSe. A Xenon lamp (MVL-210, Optpe, Japan) was used as the irradiation light source. Before the measurements, the prepared products were placed in a vacuum tube and pretreated for 1 h at 200 °C.
DFT calculations
A DFT computational study of the electronic structures was carried out using a Simulation Package (VASP). The VASP package implemented DFT in the Kohn–Sham formulation using a plane wave basis and the projector-augmented wave formalism (PAW). Bi-6p, Cl-3p, and O-2p electrons were treated as the valence electrons in the PAW potentials. A 2 × 2 × 2 supercell of monoclinic phase BiOCl was considered in the calculations. All atoms were fully relaxed for structural relaxation until the atomic forces were smaller than 0.01 eV Å−1 on each atomic site. The energy cut-off of the plane wave basis set was 500 eV. 2 × 2 × 2 k-points were employed in the calculations. For the structure containing an O defect, an oxygen atom was removed from the BiOCl supercell. For the H-addition simulations a hydrogen atom was added into the BiOCl OV supercell.
Syntheses and materials
Details regarding the chemicals and gases used, as well as catalyst syntheses are included in the Supplementary Methods.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information, or from the corresponding author upon reasonable request.
References
Takanabe, K. Photocatalytic water splitting: quantitative approaches toward photocatalyst by design. ACS Catal. 7, 8006–8022 (2017).
Wu, J. et al. Efficient visible-light-driven CO2 reduction mediated by defect-engineered BiOBr atomic layers. Angew. Chem. Int. Ed. 57, 8719–8723 (2018).
Li, X. et al. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 4, 690–699 (2019).
Li, X. et al. Ultrathin conductor enabling efficient IR light CO2 reduction. J. Am. Chem. Soc. 141, 423–430 (2019).
Wang, S. et al. In situ carbon homogeneous doping on ultrathin bismuth molybdate: a dual-purpose strategy for efficient molecular oxygen activation. Adv. Funct. Mater. 27, 1703923 (2017).
Chen, X. et al. Properties of disorder-engineered black titanium dioxide nanoparticles through hydrogenation. Sci. Rep. 3, 1510 (2013).
Li, J. et al. Superior visible light hydrogen evolution of Janus bilayer junctions via atomic-level charge flow steering. Nat. Commun. 7, 11480 (2016).
Liang, L. et al. Infrared light-driven CO2 overall splitting at room temperature. Joule 2, 1004–1016 (2018).
Chen, X. et al. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 311, 746–750 (2018).
Feng, H. et al. Activating titania for efficient electrocatalysis by vacancy engineering. ACS Catal. 8, 4288–4293 (2018).
Kobayashi, Y. et al. An oxyhydride of BaTiO3 exhibiting hydride exchange and electronic conductivity. Nat. Mater. 11, 507–11 (2012).
Lin, C. et al. Hydrogen-incorporated TiS2 ultrathin nanosheets with ultrahigh conductivity for stamp-transferrable electrodes. J. Am. Chem. Soc. 135, 5144–51 (2013).
Wang, G. et al. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 11, 3026–33 (2011).
Liu, Y. et al. The origin of enhanced photocatalytic activities of hydrogenated TiO2 nanoparticles. Dalton Trans. 46, 10694–10699 (2017).
Gurylev., V., Su., C.-Y. & Perng, T.-P. Hydrogenated ZnO nanorods with defect-induced visible light-responsive photoelectrochemical performance. Appl. Surf. Sci. 411, 279–284 (2017).
Varley, J. B. et al. Hydrogenated cation vacancies in semiconducting oxides. J. Phys. Condens Matter 23, 334212 (2011).
Szûcs, B. et al. Physics and chemistry of hydrogen in the vacancies of semiconductors. Phys. Rev. B 68, 085202 (2003).
Jalan, B. et al. Effects of hydrogen anneals on oxygen deficient SrTiO3−x single crystals. Appl. Phys. Lett. 93, 052907 (2008).
Iwazaki, Y., Suzuki, T. & Tsuneyuki, S. Negatively charged hydrogen at oxygen-vacancy sites in BaTiO3: density-functional calculation. J. Appl. Phys. 108, 083705 (2010).
Janotti, A. & Van de Walle, C. G. Hydrogen multicentre bonds. Nat. Mater. 6, 44–7 (2007).
Van de Walle, C. G. & Neugebauer, J. Hydrogen in semiconductors. Annu. Rev. Mater. Sci. 36, 179–198 (2006).
Li, H. et al. Oxygen vacancy structure associated photocatalytic water oxidation of BiOCl. ACS Catal. 6, 8276–8285 (2016).
Ma, Z. et al. Oxygen vacancies induced exciton dissociation of flexible BiOCl nanosheets for effective photocatalytic CO2 conversion. J. Mater. Chem. A 5, 24995–25004 (2017).
Cui, D. et al. Band-gap engineering of BiOCl with oxygen vacancies for efficient photooxidation properties under visible-light irradiation. J. Mater. Chem. A 6, 2193–2199 (2018).
Mao, C. et al. Visible light driven selective oxidation of amines to imines with BiOCl: does oxygen vacancy concentration matter? Appl. Catal. B 228, 87–96 (2018).
Zhu, J. et al. Intrinsic defects and H doping in WO3. Sci. Rep. 7, 40882 (2017).
Ye, L. et al. Synthesis of black ultrathin BiOCl nanosheets for efficient photocatalytic H2 production under visible light irradiation. J. Power Sources 293, 409–415 (2015).
Wang, Y. et al. Single-atomic Cu with multiple oxygen vacancies on Ceria for electrocatalytic CO2 reduction to CH4. ACS Catal. 8, 7113–7119 (2018).
Liu, F. et al. Transfer channel of photoinduced holes on a TiO2 surface as revealed by solid-state nuclear magnetic resonance and electron spin resonance spectroscopy. J. Am. Chem. Soc. 139, 10020–10028 (2017).
Mehta, M. et al. Hydrogen treated anatase TiO2: a new experimental approach and further insights from theory. J. Mater. Chem. A 4, 2670–2681 (2016).
Yu, G. et al. Dehydration and dehydroxylation of layered double hydroxides: new insights from solid-state NMR and FT-IR studies of deuterated samples. J. Phys. Chem. C 119, 12325–12334 (2019).
Zhu, G. et al. Hydrogenated blue titania with high solar absorption and greatly improved photocatalysis. Nanoscale 8, 4705–12 (2016).
Crocker, Mark et al. 1H NMR spectroscopy of titania. J. Chem. Soc. 92, 2791–2798 (1996).
Wang, L. et al. Boosting visible-light-driven photo-oxidation of BiOCl by promoted charge separation via vacancy engineering. ACS Sustain. Chem. Eng. 7, 3010–3017 (2019).
Tian, Y. et al. BiOCl nanowire with hierarchical structure and its Raman features. Appl. Surf. Sci. 258, 1949–1954 (2012).
He, W. et al. Defective Bi4MoO9/Bi metal core/shell heterostructure: enhanced visible light photocatalysis and reaction mechanism. Appl. Catal. B 239, 619–627 (2018).
Li, J. et al. Synergistic integration of Bi metal and phosphate defects on hexagonal and monoclinic BiPO4: enhanced photocatalysis and reaction mechanism. Appl. Catal. B 243, 313–321 (2019).
Wang, H. et al. Unraveling the mechanisms of visible light photocatalytic NO purification on earth-abundant insulator-based core-shell heterojunctions. Environ. Sci. Technol. 52, 1479–1487 (2018).
Wang, H. et al. Highly enhanced visible light photocatalysis and in situ FT-IR studies on Bi metal@defective BiOCl hierarchical microspheres. Appl. Catal. B 225, 218–227 (2018).
Debeila, M. A., Coville, N. J., Scurrell, M. S. & Hearne, G. R. The effect of calcination temperature on the adsorption of nitric oxide on Au-TiO2: drifts studies. Appl. Catal. A 291, 98–115 (2005).
Li, H. et al. Oxygen vacancies mediated complete visible light NO oxidation via side-on bridging superoxide radicals. Environ. Sci. Technol. 52, 8659–8665 (2018).
Hadjiivanov, K. & Knözinger, H. Species formed after NO adsorption and NO + O2 co-adsorption on TiO2: an FTIR spectroscopic study. Phys. Chem. Chem. Phys. 2, 2803–2806 (2017).
Huo, W. C. et al. Synthesis of Bi2WO6 with gradient oxygen vacancies for highly photocatalytic NO oxidation and mechanism study. Chem. Eng. J. 361, 129–138 (2017).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 51672018, 11874003), and the Academic Excellence Foundation of BUAA for Ph.D. Students, Fundamental Research Fund for Centre University and Australian Research Council (ARC) Centre of Excellence Scheme (CE140100012&DP170102267 &DP170101467). The authors would like to thank the scholarship support from China Scholarship Council (CSC).
Author information
Authors and Affiliations
Contributions
D.C., W.H., Y.D. and J.C. conceived and designed the research. D.C. performed the synthesis and carried out all the catalytic tests. K.X. contributed to DFT calculations. D.L. performed the NMR test. X.D. and F.D. performed the in situ FTIR test. All authors participated in drafting the paper and gave approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cui, D., Xu, K., Dong, X. et al. Controlled hydrogenation into defective interlayer bismuth oxychloride via vacancy engineering. Commun Chem 3, 73 (2020). https://doi.org/10.1038/s42004-020-0319-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-020-0319-9
This article is cited by
-
Photochemical reactor for selective hydrogenation of asphaltene molecules at room temperature in absence of a catalyst
Brazilian Journal of Chemical Engineering (2023)
-
S vacant CuIn5S8 confined in a few-layer MoSe2 with interlayer-expanded hollow heterostructures boost photocatalytic CO2 reduction
Rare Metals (2022)
-
Broadband dielectric spectroscopy for monitoring temperature-dependent chloride ion motion in BiOCl plates
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.