Abstract
Cesium lead halide perovskite (CsPbX3, X=Cl, Br, I) nanocrystals embedded in Cs4PbX6 or CsPb2X5 matrices have received interests due to their excellent optical properties. However, their precise endotaxial structures are not known, and the origin of photoluminescence remains controversial. Here we report a sonochemistry technique that allowed us to synthesize high-quality CsPbBr3-based microcrystals in all ternary phases, simply by adjusting precursor concentrations in a polar aprotic solvent, N,N-dimethylformamide. The microcrystals with diverse morphologies enabled us to visualize the lattice alignments in the dual-phase composites and confirm CsPbBr3 nanocrystals being the photoluminescent sites. We demonstrate high solid-state quantum yield of >40% in Cs4PbBr6/CsPbBr3 and lasing of CsPbBr3 microcrystals as small as 2 µm in size. Real-time optical analysis of the reaction solutions provides insights into the formation and phase transformation of different CsPbBr3-based microcrystals.
Similar content being viewed by others
Introduction
Three-dimensional (3D) lead halide perovskites (LHPs) with the form of APbX3 (A=Cs+, CH3NH3+, X=Cl−, Br−, I−) are promising optical materials. These materials offer a long carrier lifetime (>1 μs), long exciton diffusion length1,2 (>1 μm), large optical cross-sections3 (~10−13 cm2), and defect tolerance owing to the antibonding character of the conduction and valence bands4. These properties make them an attractive building block for solar cells, light-emitting diodes, and lasers5. Among various types of LHPs, all-inorganic CsPbBr3 received increasing interest due to their high luminescent quantum yields in solid states in the green range and superior environmental stability to other perovskites with organic cations. CsPbBr3 has two lower-dimensional counterparts: zero-dimensional (0D) Cs4PbBr6 and quasi-two-dimensional (2D) CsPb2Br5. They represent different ternary phases of the Cs–Pb–Br compounds and can be formed from precursors, such as CsBr and PbBr2 (Supplementary Fig. 1)6. Cs4PbBr6 and CsPb2Br5 are known to have large bandgap energies of 3.7 eV and 3.1 eV, respectively7,8. Nonetheless, some confusion has arisen when these non-perovskite materials were claimed to generate green photoemission4,9. Recent studies7,10,11,12 have suggested that these materials contain CsPbBr3 nanocrystals (NCs) that are responsible for photoluminescence.
We refer these dual-phase materials to as Cs4PbBr6/CsPbBr3 (CsPbBr3 NCs in a Cs4PbBr6 matrix) and CsPb2Br5/CsPbBr3 (CsPbBr3 NCs in a CsPb2Br5 matrix). While much progress in the dual-phase materials is expected, it has been difficult to visualize the relative lattice orientation between LHPs NCs and the host matrix clearly by using HRTEM, due to difficulties such as the low damage threshold of the materials by electron beam and the inadequate sample sizes being too small lateral size (<10 nm) to observe more than four different lattice planes at given electron beam or too large in thickness (larger than few hundreds of nm) in thickness to get clear images7,12,13,14.
Herein, we report a new method based on sonochemistry that enables a facile, rapid synthesis of various phase, and dimensional CsPbBr3 perovskites microcrystals in a polar aprotic solvent, N,N-dimethylformamide. We show this technique can produce both types of dual-phase materials, as well as single-phase LHP microcrystals, with various surface morphologies depending on precursor concentrations. Our investigation provides insights into the formation kinetics and phase transition of the Cs–Pb–Br compounds. The produced microparticles enabled us to investigate the lattice structures and optical properties of the various CsPbBr3-based compounds.
Results and discussion
Sonochemical synthesis of various LHPs
The general scheme of sonochemical synthesis starts with placing two precursor salts, CsBr and PbBr2, with sufficient quantities beyond their maximum soluble amount in a polar aprotic solvent. For CsBr and PbBr2 salts, N,N-dimethylformamide (DMF) produced high-quality CsPbBr3 microparticles among different polar aprotic solvents having similar dipole moments (acetone, ethyl acetate (EtoAC), γ-butyrolactone (GBL), and dimethyl sulfoxide (DMSO)) (Supplementary Fig. 2). So, all the experiments presented herein after were obtained using DMF. As illustrated in Fig. 1a, after several minutes of ultrasonication at room temperature (Supplementary Fig. 3), the salts are fully dissolved and produce a stable solution. The final solution varies in color, depending on the ratio of the starting concentration of the precursors (Supplementary Fig. 4).
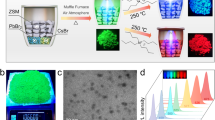
a Schematic of the sonochemical synthesis using ultrasonication of CsBr and PbBr2 salts in DMF. The starting concentration of the precursor materials are denoted as “a” and “b”. b Two-dimensional concentration phase diagram of the sonochemical reaction products. SEM images of dual-phase Cs4PbBr6/CsPbBr3 (hexagonal plate, microdisc, and rhombus), single-phase Cs4PbBr6 (granular prism), single-phase CsPbBr3 (cuboid), dual-phase CsPb2Br5/CsPbBr3 (truncated octahedron, cuboctahedron, wedding cake, and fibrous). Scale bars, 2 μm.
Figure 1b summarizes our finding arranged in a two-dimensional phase diagram, along with the SEM images of various distinct types of microparticles formed. We used parameters “a” and “b” to denote the concentrations of the CsBr and PbBr2 precursors, respectively. The values are normalized to 75 mM (e.g., “a = 1” corresponds to 75 mM of CsBr, and “b = 2” refers to 150 mM of PbBr2).
From stoichiometry, the ideal concentration ratio to produce CsPbBr3 would be a/b = 1, if the two precursor materials were equally dissolved in the solvent. Experimentally, CsPbBr3 microcrystals were produced when the ratio a/b was approximately between 0.8 and 2, and both “a” and “b” are in a range from 1 to 4 (i.e., 75–300 mM). The orange-color solution obtained after ultrasonication contains single-phase CsPbBr3 microparticles with the cuboidal shape (Supplementary Fig. 5).
When a/b <0.8 and b >1, the product of reaction is dual-phase CsPb2Br5/CsPbBr3 composites, which show intense yellow color under room light. Their surface morphology varied depending on the precursor concentration (Supplementary Fig. 6). For a = 1 (75 mM) and b = 2 or 3 (75 or 150 mM), the microparticles have largely octahedral shapes. With b = 4 (300 mM), the particles tend to have irregular shapes and rough surfaces, probably resulting from rapid surface nucleation due to the high PbBr2 concentration. No crystals were formed at low concentrations of a = 0.5 (37.5 mM) and b = 1 (75 mM).
When a/b >2 and a >2 (150 mM), we found that single-phase CsPbBr3 microcrystals are initially formed, but converted to white-color Cs4PbBr6 microcrystals and then to lemon-color dual-phase Cs4PbBr6/CsPbBr3 composites. Cs4PbBr6/CsPbBr3 composites are found in a mixture of rhombus and hexagonal plates (Supplementary Fig. 7). When these Cs4PbBr6/CsPbBr3 plates are left for about 1 h in the solution with ultrasonication being off, they undergo phase transition to Cs4PbBr6/CsPbBr3 with micro-discoidal shapes. The same morphological transition was observed when the solution was vigorously shaken by hands. This dynamic process is described later in more detail.
When a/b = 2 and a <= 2 (150 mM), CsPbBr3 microparticles are initially formed and converted to Cs4PbBr6 microcrystals, and they remain as the final product. Single-phase Cs4PbBr6 microcrystals have a granular structure (Supplementary Fig. 8).
Structures of dual-phase LHPs
To identify the crystal structure and stoichiometry of the various products, we performed powder X-ray diffraction (PXRD) and energy dispersive X-ray spectroscopy (EDS). The data (Supplementary Figs. 9–12) confirmed the orthorhombic structure of CsPbBr3 (space group Pbnm, A = 8.20 Å, B = 8.24 Å, C = 11.74 Å), the trigonal structure of Cs4PbBr6 in the single- and dual-phase Cs4PbBr6 products (R\(\bar 3\)c, A = B = 13.73 Å, C = 17.32 Å), and the tetragonal structure of CsPb2Br5 in the dual-phase composite (I4/mcm, A = B = 8.45 Å, C = 15.07 Å). These results are consistent with previous reports8,15,16.
Unlike previous dual-phase materials7,12,13,14, structurally anisotropic microparticles produced by sonochemistry were well suited to obtain high-quality HRTEM having more than four different lattice planes at given the direction of an electron beam. For HRTEM imaging, we used micro-discoidal Cs4PbBr6/CsPbBr3 (a = 3 (225 mM), b = 1 (75 mM)) having average thickness of 80 nm (N = 15) and wedding-cake CsPb2Br5/CsPbBr3 (a = 1 (75 mM), b = 4 (300 mM)) consisting of multiple thin layers having average thickness of 50 nm (N = 15) (Supplementary Fig. 13).
The real-space images and the corresponding fast Fourier transform (FFT) analysis revealed the relative lattice orientation of the endotaxial structures (Fig. 2a, c; Supplementary Fig. 14). In the case of Cs4PbBr6/CsPbBr3, well-defined lattice fringes with 3.7 Å and 4.2 Å having intersection angle of 42° are indexed to (212) and (210) of the Cs4PbBr6 matrix, and higher-contrast lattice fringes with 3.7 Å and 3.5 Å having intersection angle of 67° are indexed to (210) and (021) of the CsPbBr3 NCs. This suggests that the [210] axis of Cs4PbBr6 is tilted by 6° with respect to the [021] axis of CsPbBr3. For CsPb2Br5/CsPbBr3, low-contrast lattice fringes with 2.6 Å and 5.8 Å having intersection angle of 35° are indexed to (222) and (110) of the CsPb2Br5 matrix, and lattice fringes in darker sub-regions with 6.8 Å and 11.6 Å having intersection angle of 35° are indexed to (210) and (021) of the CsPbBr3 NCs. Hence, the [110] axis of CsPb2Br5 is aligned to the [1\(\bar 2\)1] axis of CsPbBr3. A computational model based on the data confirmed good facet matching between CsPbBr3 NCs and non-perovskite matrices (Fig. 2b, d). From the TEM images, we determined the effective size of CsPbBr3 NCs embedded in the matrices by measuring the diameter of the largest circle circumscribing the NCs (Supplementary Fig. 15). The CsPbBr3 NCs in Cs4PbBr6 have sizes of 3–5 nm with a mean effective diameter of 4.2 nm. The CsPbBr3 NCs in CsPb2Br5 are larger, ranging from 10 to 20 nm, with a mean effective diameter of 14.6 nm.
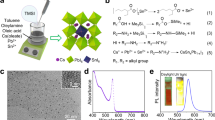
a HRTEM image of a dual-phase Cs4PbBr6/CsPbBr3 microdisc. b Cs4PbBr6 and CsPbBr3 crystal structure based on the HRTEM image. c HRTEM image of a dual-phase CsPb2Br5/CsPbBr3 wedding-cake crystal. d CsPb2Br5 and CsPbBr3 crystal structure based on the HRTEM image. e–g Absorbance (dashed orange lines) and fluorescence (solid green lines; excitation at 480 nm) spectra of CsPbBr3, Cs4PbBr6/CsPbBr3, and CsPb2Br5/CsPbBr3. h Time-resolved photoluminescence measurement. The measured data (circles) are fitted with triple exponential curves (lines).
Optical properties of dual-phase LHPs
Using a custom-built microscope coupled with a grating-based spectrometer (Supplementary Fig. 16), we measured the optical emission and absorption spectra of various product particles either in solution (Fig. 2e–g). The optical spectra did not change after the particles have been transferred to a glass substrate. CsPbBr3 microcrystals have an absorption edge at 538 nm (2.31 eV), weak excitonic peak at 523 nm, and low Urbach energy of 23 meV. Dual-phase Cs4PbBr6/CsPbBr3 microcrystals have an absorption edge at 525 nm (2.37 eV), and their fluorescence peaks are blue-shifted to 512 nm. The magnitude of blue shift varied between 10 and 21 nm, depending on the precursor ratio and the conversion method (Supplementary Fig. 17). The bandgap changes, ΔE, by quantum confinement is given by:
where \(m_h^ \ast = 0.14\) and \(m_e^ \ast = 0.15\) denote the effective mass of the hole and electron17, respectively, in CsPbBr3 in the unit of the electronic mass, d the diameter of a spherical potential well, ε the permittivity of the matrix surrounding CsPbBr3 NCs. CsPbBr3 NCs. ε/ε0 = 3.1 was calculated for Cs4PbBr6 by a density functional theory18. The spectral shifts we measured from the spectra indicate d = 6.2 nm and 5.6 nm, respectively. These values are reasonable, but larger than the mean diameter of 4.2 nm. The discrepancy may be attributed to the nonspherical shapes of the nanocrystals and interfacial effects with the Cs4PbBr6 matrix. The dielectric constant of CsPb2Br5 matrix is unknown. Assuming it is the same as Cs4PbBr6, the quantum confinement effect for NCs with the mean diameter of 14.6 nm is estimated to be −6 meV. The fluorescence peak of CsPb2Br5/CsPbBr3 microparticles is at 530 nm, ~1 nm shifted from the 531 nm peak of CsPbBr3 microcrystals. This shift of ~4.4 meV corresponds to d = 11 nm.
We investigated time-resolved photoluminescence using a picosecond frequency-doubled laser (λ = 382 nm). The experimental time-resolved photoluminescence data (Fig. 2h; Supplementary Table 1) were fitted to a three-exponential decay curve:
where A1, A2, and A3 are pre-exponential factors, and τ1, τ2, and τ3 are lifetime constants. The total decay time was computed from weighted lifetime constants:
The radiative decay time of the sample is related to total decay time and absolute PLQY:
Dual-phase Cs4PbBr6/CsPbBr3 has a much faster radiative lifetime of 9.7 ns, compared with the lifetime of 1.2 μs for single-phase CsPbBr3 and 4.4 µs for dual-phase CsPb2Br5/CsPbBr3. Cs4PbBr6/CsPbBr3 has a high photoluminescence quantum yield (PLQY) of over 40% due to both the quantum confinement and low dielectric constant of Cs4PbBr613.
Lasing of optically pumped LHP microcrystals
Single-mode lasing from single-phase CsPbBr3 microcrystals was observed when excited by nanosecond-pulsed optical pumping at 480 nm (Fig. 3; Supplementary Fig. 18). The smallest size of lasing CsPbBr3 microcrystals was 2 µm (Fig. 3b; Supplementary Fig. 18). The laser emission linewidth was ~0.2 nm above a threshold pump energy of 1.7 mJ/cm2, and the spontaneous emission factor (β) was 0.05 (Fig. 3c). The other device presents the laser emission linewidth of 0.3 nm above a threshold pump energy of 2.2 mJ/cm2, and the spontaneous emission factor was 10−3 (Supplementary Fig. 18). The converted threshold pump energy to threshold carrier density is ~3 × 1019 cm−3, which is higher than theoretical estimation of Mott density (1018 cm−3)19. This infers lasing in the electron hole plasma state (EHP), rather than excitonic state5,19, which is beneficial to build up large population inversion via bandgap renormalization (BGR)20. The laser emission of single-phase CsPbBr3 microparticles in air (15 samples) at a pump fluence twice the lasing threshold was prolonged for 105 pump pulses (5000 s at 20 Hz) with a pulse-to-pulse wavelength fluctuation of 0.47 nm (Supplementary Fig. 18). On the contrary, dual-phase microparticles did not support laser oscillation even at higher pump energy levels up to tens of mJ/cm2. Considering the high PLQY of CsPbBr3 NCs in the dual-phase composites, we attribute the failure to reach lasing threshold to the weaker cavity resonance due to the lower refractive index (~1.8) of the Cs4PbBr621 and CsPb2Br5 matrices compared with the index (~2.6) of CsPbBr35, and relatively small amount of the optical gain in the excitonic state due to un-normalized bandgap.
a Output spectra from a 2-μm sized CsPbBr3 microcrystal upon nanosecond optical pumping (480 nm) below and above lasing threshold. b Wide-field fluorescence images below and above laser threshold. Scale bar, 2 μm. c A light-in–light-out curve, showing a threshold pump fluence of 1.7 mJ/cm2 and a spontaneous emission factor (β) of ~0.05.
Mechanism of dual-phase formation
To gain insights into the mechanism of dual-phase formation, we investigated distinct intermediate reaction steps, which involves color changes of the solution. The sonochemical synthesis of Cs4PbBr6/CsPbBr3 composite is comprised six distinct steps: the formation of orange-color CsPbBr3 (steps i–iv), the phase transformation from orange-color CsPbBr3 to white-color single-phase Cs4PbBr6 (step v), and the formation of dual-phase Cs4PbBr6/CsPbBr3 (step vi) (Fig. 4a; Supplementary Movie 1).
a Time-lapse change of precursor solution (a = 3, b = 1) during ultrasonication and a schematic of six reaction intermediate steps. b (a–c) The temperature dependence of the reaction rate, v [s−1], for each reaction step (ii to iv); and (d) a reaction coordinate diagram of the sonochemical synthesis of CsPbBr3 microcrystals. c Bright-field images showing the phase transformation of single-phase CsPbBr3 cuboids to single-phase Cs4PbBr6 microdiscs. d The color and photoluminescence of solutions immediately after ultrasonication and after 12-h incubation under room light and UV light. e Bright-field and fluorescence images of dual-phase Cs4PbBr6/CsPbBr3 microdiscs.
Our interpretation of the process is as follows. When CsBr and PbBr2 salts were mixed with DMF with concentration of a = 3 and b = 1, orange-color CsPbBr3 layer is immediately formed via interfacial conversion on the surface of undissolved salts [step i]. The ultrasonic pressure and temperature modulation allows the remaining salts to be completely dissolved [step ii]. Simultaneously, the reaction intermediate, \({\mathrm{PbBr}}_4^{2 - }\), increases via:
Since the reaction species are optically transparent, the solution turns clear. The spontaneous nucleation and growth of CsPbBr3 occur when the concentration of \({\mathrm{PbBr}}_4^{2 - }\) reaches the level of saturation, at which the solution turns orange. The crystallization reaction may be described as:
We measured the time trace of the color intensity (steps i–iv) (Supplementary Fig. 19), and calculated the reaction rates from the slope and duration of the color intensity profile. Figure 4b shows logarithmic plots of the reaction rates, v, as a function of the reciprocal of temperature T, and an overall reaction coordinate diagram. From the curve fitting of the data with the Arrhenius and Eyring equation, we obtained Ea = −35 kJ/mol for step (ii), 27 kJ/mol for step (iii), and 23 kJ/mol for step (iv). The negative activation energy and exothermic dissolution of bulk CsPbBr3 in step (ii) are due to low lattice-formation energy of CsPbBr3. This low energy barrier is a double-edged sword making LHPs easy to be crystallized and degraded22. The measured activation energies of the step (iii) and step (iv) are approximately three times smaller than that of conventional thin film formation (86 kJ/mol)23. The reduced activation energy likely comes from the vibrant oscillation of the pressure and temperature in ultrasonication microbubbles24. This low activation energy is a key to the rapid synthesis.
To understand the transformation from intermediate single-phase CsPbBr3 to single-phase Cs4PbBr6, we stopped ultrasonication after 10 min right after step (iv), transferred a titer amount of the solution onto a glass substrate and examined the sample using bright-field optical microscopy (Fig. 4c). Under the microscope, we observed that Cs4PbBr6 microdiscs appeared as CsPbBr3 microcuboids were dissolving (Supplementary Movie 2). The formation of Cs4PbBr6 can be described as:
The fluorescence quantum yield of the microdiscs is nearly zero immediately after their formation, but gradually increases over time (Fig. 4d). This is due to the conversion of single-phase Cs4PbBr6 to dual-phase Cs4PbBr6/CsPbBr3 (step vi). This conversion occurs spontaneously at room temperature, but at a much slower speed over 12 h. The final Cs4PbBr6/CsPbBr3 microdiscs emit bright fluorescence (Fig. 4e). The conversion of single-phase Cs4PbBr6 microdiscs proceeds with slow self-formation of CsPbBr3 NCs in the Cs4PbBr6 matrix, releasing CsBr to the solution, via:
where x («1) denotes the amount of conversion. A similar CsBr extraction process has previously been observed during the evolution of single-phase Cs4PbBr6 to single-phase \({\mathrm{CsPbBr}}_{3}\)7,25.
The sonochemical synthesis of dual-phase CsPb2Br5/CsPbBr3 composite (a = 1, b = 3) appeared to be straightforward without producing any apparent intermediates. Time-lapse video (Supplementary Fig. 20, Supplementary Movie 3) shows that as soon as the precursor salts are placed in DMF, orange-color CsPbBr3 layers are formed on the surface of the salts. After 2 min of ultrasonication, the entire solution turns yellow as CsPb2Br5/CsPbBr3 microparticles are produced. This fast and simple formation is in contrast to the slow formation of Cs4PbBr6/CsPbBr3. Considering the tetragonal structure of CsPb2Br5 with alternating Cs+ and \({\mathrm{Pb}}_2{\mathrm{Br}}_5^ -\) layers, the formation mechanism may be described as:
During the growth of CsPb2Br5, the self-formation of CsPbBr3 NCs in the CsPb2Br5 matrix simultaneously occurs by releasing PbBr2 to the solution:
The release of PbBr2 during the self-formation of NCs promotes the formation of the CsPb2Br5 matrix.
In summary, we have shown that the sonochemical processes led to rapid synthesis of dual-phase perovskites. Both Cs4PbBr6/CsPbBr3 and CsPb2Br5/CsPbBr3 composites have well-defined endotaxy structures with good lattice matching between embedded CsPbBr3 NCs and the non-luminescent matrices4,9,26. The high solid-state PLQY of >40% in Cs4PbBr6/CsPbBr3 and efficient lasing from single CsPbBr3 microparticles as small as 2 µm attest the high quality of the microcrystals. Lastly, our real-time measurement data suggest that CsPbBr3 NCs in the Cs4PbBr6 or CsPb2Br5 matrix is formed via a partial extraction of CsBr or PbBr2. Single- and dual-phase CsPbBr3-based microparticles may prove to be useful building blocks for optical devices.
Methods
Chemicals and reagents
CsBr (99.99%), PbBr2 (99.99%), N,N-dimethylformamide (anhydrous, 99.8%), acetone (99.9%), ethyl acetate (EtoAC) (anhydrous, 99.8%), γ-butyrolactone (GBL) (99.9%), and dimethyl sulfoxide (DMSO) (anhydrous, 99.9%) were purchased from Sigma-Aldrich. All reagents were used as received from Sigma-Aldrich without further purification.
Sonochemical synthesis of single-phase perovskite microcrystals
For producing inorganic perovskite CsPbBr3, CsBr and PbBr2 were dispersed at an equal concentration in 1 mL of N,N-dimethylformamide (DMF) in a vial. The typical concentration was 0.075 M (i.e., a = 1 and b = 1) or its multiples up to 0.3 M (a = b = 2, 3, or 4). The vial was placed into a bath-type ultrasonicator (Elmasonic P60H, Elma) or a single-step tip ultrasonicator (Fisherbrand Q125) in room temperature and irradiated with ultrasonic waves (frequency: 20 kHz∼80 kHz). After 2–3 min of ultrasonication, single-phase CsPbBr3 microcrystals were spontaneously crystallized and dispersed in the solution.
Sonochemical synthesis of dual-phase Cs4PbBr6/CsPbBr3 microcrystals
Ultrasonication of 1 mL of DMF solution of CsBr (0.225 M or 0.3 M) and PbBr2 (0.075 M) for 2 min yields single-phase CsPbBr3 microcrystals. Continuing ultrasonication for additional several min makes the orange-colored solution to white, opaque dispersion of single-phase Cs4PbBr6 microparticles, and then to lemon-colored solution of dual-phase Cs4PbBr6/CsPbBr3 microparticles. This process takes about 13–15 min. After the ultrasonication has been stopped, the color of the solution becomes gradually brighter at room temperature overnight. The morphologies of the final particles are a mixture of hexagon and rhombus. As an alternative way to synthesize dual-phase Cs4PbBr6/CsPbBr3 microparticles, after the synthesis of CsPbBr3 microcrystals by 2 min of ultrasonication, the solution is removed from the ultrasonicator, and then vigorous shaking is applied for 1 h until the color turns to light green. Likewise, the lemon color becomes gradually intense over time in room temperature.
Sonochemical synthesis of single-phase Cs4PbBr6 microcrystals
For single-phase Cs4PbBr6, CsBr (0.075 M), and PbBr2 (0.0375 M) in 1 mL of DMF were used as a precursor solution. In the case of CsBr (0.15 M) and PbBr2 (0.075 M), a mixture of single-phase CsPbBr3 particles and Cs4PbBr6 particles were formed.
Sonochemical synthesis of dual-phase CsPb2Br5/CsPbBr3 microcrystals
Dual-phase CsPb2Br5/CsPbBr3 were obtained when the concentration of PbBr2 was higher than the concentration of CsBr. Regular truncated octahedron morphology was obtained with CsBr (0.075 M) and PbBr2 (0.15 M). Cuboctahedron particles were obtained with CsBr (0.075 M) and PbBr2 (0.225 M). For wedding cake or fibrous structure with rough surface, CsBr (0.075 M) and PbBr2 (0.3 M) were used.
Structural characterization
For SEM and EDX measurements, LHPs microcrystals were transferred onto a chipped Si wafer by drop-casting and imaged using a Zeiss Merlin high-resolution SEM equipped with an EDX detector operated at 15 kV. For TEM measurements, samples were prepared by drop-casting LHP microparticles onto TEM grids (Ted Pella). TEM images were acquired using a FEI Tecnai Multipurpose TEM operated at 120 kV. The illumination beam was expanded to avoid sample damage. For PXRD measurements, PXRD patterns over 2θ angles from 10° to 60° were collected using a PANalytical X’Pert PRO high-resolution X-ray diffraction system with a CuKα irradiation source. These measurements were performed at MIT Center for Material Science and Engineering (CMSE).
Optical characterization
See Supplementary Methods.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The main data supporting the finding of this study are available within the paper and its Supplementary Information file. Other relevant data are available from the corresponding author upon reasonable request.
References
Stranks, S. D. et al. Electron-hole diffusion lengths exceeding. Science 342, 341–344 (2014).
Yettapu, G. R. et al. Terahertz conductivity within colloidal CsPbBr3 perovskite nanocrystals: remarkably high carrier mobilities and large diffusion lengths. Nano Lett. 16, 4838–4848 (2016).
Yakunin, S. et al. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 6, 8056 (2015).
Akkerman, Q. A., Rainò, G., Kovalenko, M. V. & Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 17, 394–405 (2018).
Eaton, S. W. et al. Lasing in robust cesium lead halide perovskite nanowires. Proc. Natl Acad. Sci. USA 113, 1993–1998 (2016).
Cola, M., Massarotti, V., Riccardi, R. & Sinistri, C. Binary systems formed by lead bromide with (Li, Na, K, Rb, Cs and Tl) Br: a DTA and diffractometric study. Zeitschrift für Naturforsch. A 26, 1328–1332 (1971).
Chen, X. et al. Centimeter‐sized Cs4PbBr6 crystals with embedded CsPbBr3 nanocrystals showing superior photoluminescence: nonstoichiometry induced transformation and light‐emitting applications. Adv. Funct. Mater. 28, 1706567 (2018).
Dursun, I. et al. CsPb2Br5 single crystals: synthesis and characterization. ChemSusChem 10, 3746–3749 (2017).
Akkerman, Q. A., Abdelhady, A. L. & Manna, L. Zero-dimensional cesium lead halides: history, properties, and challenges. J. Phys. Chem. Lett. 9, 2326–2337 (2018).
Qin, Z. et al. Revealing the origin of luminescence center in 0D Cs4PbBr6 perovskite. Chem. Mater. 31, 9098–9104 (2019).
Wang, C. et al. Extrinsic green photoluminescence from the edges of 2D cesium lead halides. Adv. Mater. 31, 1902492 (2019).
Quan, L. N. et al. Highly emissive green perovskite nanocrystals in a solid state crystalline matrix. Adv. Mater. 29, 1605945 (2017).
Xu, J. et al. Imbedded nanocrystals of CsPbBr3 in Cs4PbBr6: kinetics, enhanced oscillator strength, and application in light‐emitting diodes. Adv. Mater. 29, 1703703 (2017).
Zhang, X. et al. All‐inorganic perovskite nanocrystals for high‐efficiency light emitting diodes: dual‐phase CsPbBr3‐CsPb2Br5 composites. Adv. Funct. Mater. 26, 4595–4600 (2016).
Fu, Y. et al. Broad wavelength tunable robust lasing from single-crystal nanowires of cesium lead halide perovskites (CsPbX3, X=Cl, Br, I). ACS Nano 10, 7963–7972 (2016).
Saidaminov, M. I. et al. Pure Cs4PbBr6: highly luminescent zero-dimensional perovskite solids. ACS Energy Lett. 1, 840–845 (2016).
Protesescu, L. et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X=Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 15, 3692–3696 (2015).
Kang, B. & Biswas, K. Exploring polaronic, excitonic structures and luminescence in Cs4PbBr6/CsPbBr3. J. Phys. Chem. Lett. 9, 830–836 (2018).
Schlaus, A. P. et al. How lasing happens in CsPbBr3 perovskite nanowires. Nat. Commun. 10, 265 (2019).
Chernikov, A., Ruppert, C., Hill, H. M., Rigosi, A. F. & Heinz, T. F. Population inversion and giant bandgap renormalization in atomically thin WS2 layers. Nat. Photonics 9, 466 (2015).
Han, D. et al. Unraveling luminescence mechanisms in zero-dimensional halide perovskites. J. Mater. Chem. C 6, 6398–6405 (2018).
Manser, J. S., Saidaminov, M. I., Christians, J. A., Bakr, O. M. & Kamat, P. V. Making and breaking of lead halide perovskites. Acc. Chem. Res. 49, 330–338 (2016).
Moore, D. T. et al. Crystallization kinetics of organic-inorganic trihalide perovskites and the role of the lead anion in crystal growth. J. Am. Chem. Soc. 137, 2350–2358 (2015).
Haqshenas, S. R., Ford, I. J. & Saffari, N. Modelling the effect of acoustic waves on nucleation. J. Chem. Phys. 145, 24315 (2016).
Palazon, F. et al. Postsynthesis transformation of insulating Cs4PbBr6 nanocrystals into bright perovskite CsPbBr3 through physical and chemical extraction of CsBr. ACS Energy Lett. 2, 2445–2448 (2017).
Miyata, K. et al. Large polarons in lead halide perovskites. Sci. Adv. 3, e1701217 (2017).
Acknowledgements
This research was supported in part by the National Institutes of Health (grant no. DP1EB024242). S.C. acknowledges the Samsung Scholarship. Part of this work used the facilities in the Center for Materials Science and Engineering at MIT.
Author information
Authors and Affiliations
Contributions
S.C. and S.H.Y. conceived the idea and designed the experiments. S.C. performed experiments and analyzed the data. S.H.Y. supervised the project. Both authors wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cho, S., Yun, S.H. Structure and optical properties of perovskite-embedded dual-phase microcrystals synthesized by sonochemistry. Commun Chem 3, 15 (2020). https://doi.org/10.1038/s42004-020-0265-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-020-0265-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.